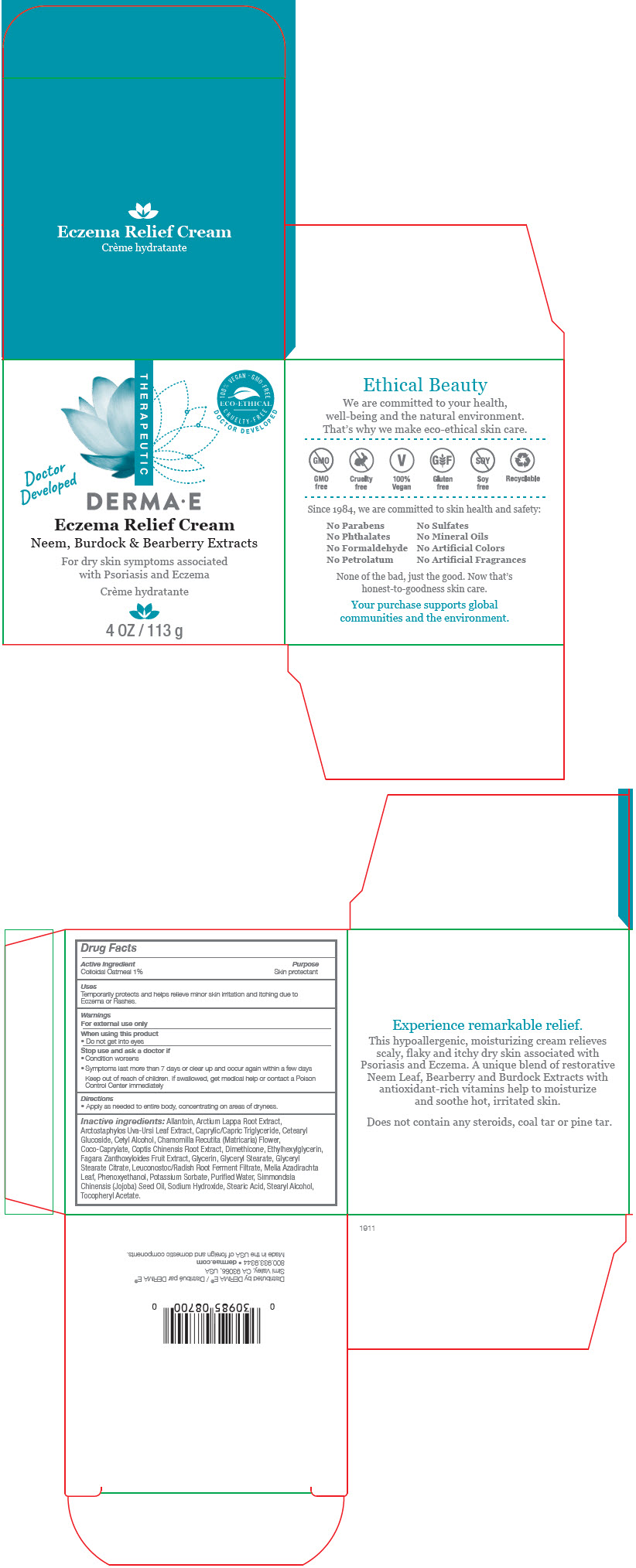
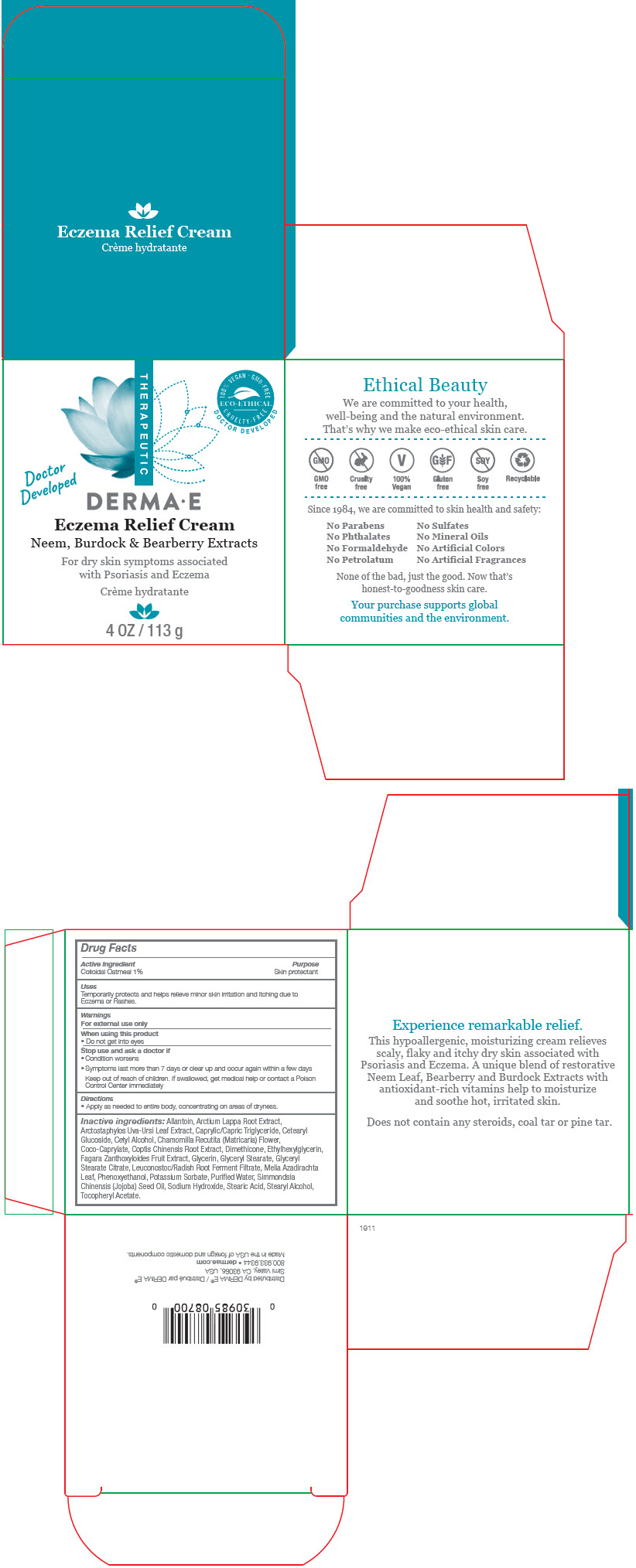
Label: DERMA-E ECZEMA RELIEF- oatmeal cream
- NDC Code(s): 51326-870-04
- Packager: Topiderm, Inc
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated August 19, 2020
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active Ingredient
- Purpose
- Uses
- Warnings
- Directions
-
Inactive ingredients
Allantoin, Arctium Lappa Root Extract, Arctostaphylos Uva-Ursi Leaf Extract, Caprylic/Capric Triglyceride, Cetearyl Glucoside, Cetyl Alcohol, Chamomilla Recutita (Matricaria) Flower, Coco-Caprylate, Coptis Chinensis Root Extract, Dimethicone, Ethylhexylglycerin, Fagara Zanthoxyloides Fruit Extract, Glycerin, Glyceryl Stearate, Glyceryl Stearate Citrate, Leuconostoc/Radish Root Ferment Filtrate, Melia Azadirachta Leaf, Phenoxyethanol, Potassium Sorbate, Purifed Water, Simmondsia Chinensis (Jojoba) Seed Oil, Sodium Hydroxide, Stearic Acid, Stearyl Alcohol, Tocopheryl Acetate.
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 113 g Jar Box
-
INGREDIENTS AND APPEARANCE
DERMA-E ECZEMA RELIEF
oatmeal creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:51326-870 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OATMEAL (UNII: 8PI54V663Y) (OATMEAL - UNII:8PI54V663Y) OATMEAL 0.01 g in 1 g Inactive Ingredients Ingredient Name Strength ALLANTOIN (UNII: 344S277G0Z) ARCTIUM LAPPA ROOT (UNII: 597E9BI3Z3) ARCTOSTAPHYLOS UVA-URSI LEAF (UNII: 3M5V3D1X36) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) CETEARYL GLUCOSIDE (UNII: 09FUA47KNA) CETYL ALCOHOL (UNII: 936JST6JCN) MATRICARIA CHAMOMILLA FLOWERING TOP (UNII: 3VNC7T6Z02) COCO-CAPRYLATE (UNII: 4828G836N6) COPTIS CHINENSIS ROOT (UNII: CXS4LJR7EL) DIMETHICONE (UNII: 92RU3N3Y1O) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) GLYCERIN (UNII: PDC6A3C0OX) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) GLYCERYL STEARATE CITRATE (UNII: WH8T92A065) LEUCONOSTOC/RADISH ROOT FERMENT FILTRATE (UNII: D2QHA03458) AZADIRACHTA INDICA LEAF (UNII: HKY915780T) PHENOXYETHANOL (UNII: HIE492ZZ3T) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) WATER (UNII: 059QF0KO0R) JOJOBA OIL (UNII: 724GKU717M) SODIUM HYDROXIDE (UNII: 55X04QC32I) STEARIC ACID (UNII: 4ELV7Z65AP) STEARYL ALCOHOL (UNII: 2KR89I4H1Y) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51326-870-04 1 in 1 BOX 09/01/2020 1 113 g in 1 JAR; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH DRUG M016 09/01/2020 Labeler - Topiderm, Inc (049121643) Registrant - Topiderm, Inc. (049121643) Establishment Name Address ID/FEI Business Operations Topiderm, Inc 049121643 MANUFACTURE(51326-870)