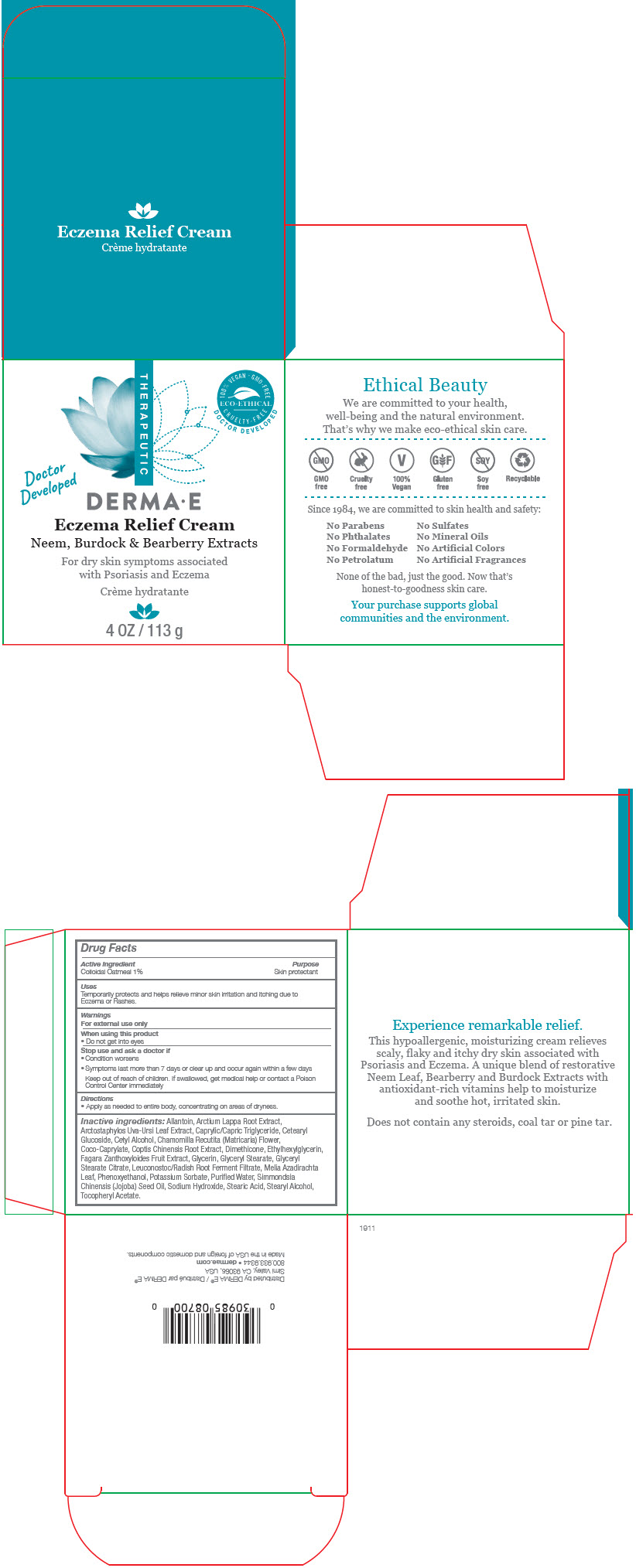
Uses
Temporarily protects and helps relieve minor skin irritation and itching due to Eczema or Rashes.
Warnings
For external use only
Inactive ingredients
Allantoin, Arctium Lappa Root Extract, Arctostaphylos Uva-Ursi Leaf Extract, Caprylic/Capric Triglyceride, Cetearyl Glucoside, Cetyl Alcohol, Chamomilla Recutita (Matricaria) Flower, Coco-Caprylate, Coptis Chinensis Root Extract, Dimethicone, Ethylhexylglycerin, Fagara Zanthoxyloides Fruit Extract, Glycerin, Glyceryl Stearate, Glyceryl Stearate Citrate, Leuconostoc/Radish Root Ferment Filtrate, Melia Azadirachta Leaf, Phenoxyethanol, Potassium Sorbate, Purifed Water, Simmondsia Chinensis (Jojoba) Seed Oil, Sodium Hydroxide, Stearic Acid, Stearyl Alcohol, Tocopheryl Acetate.