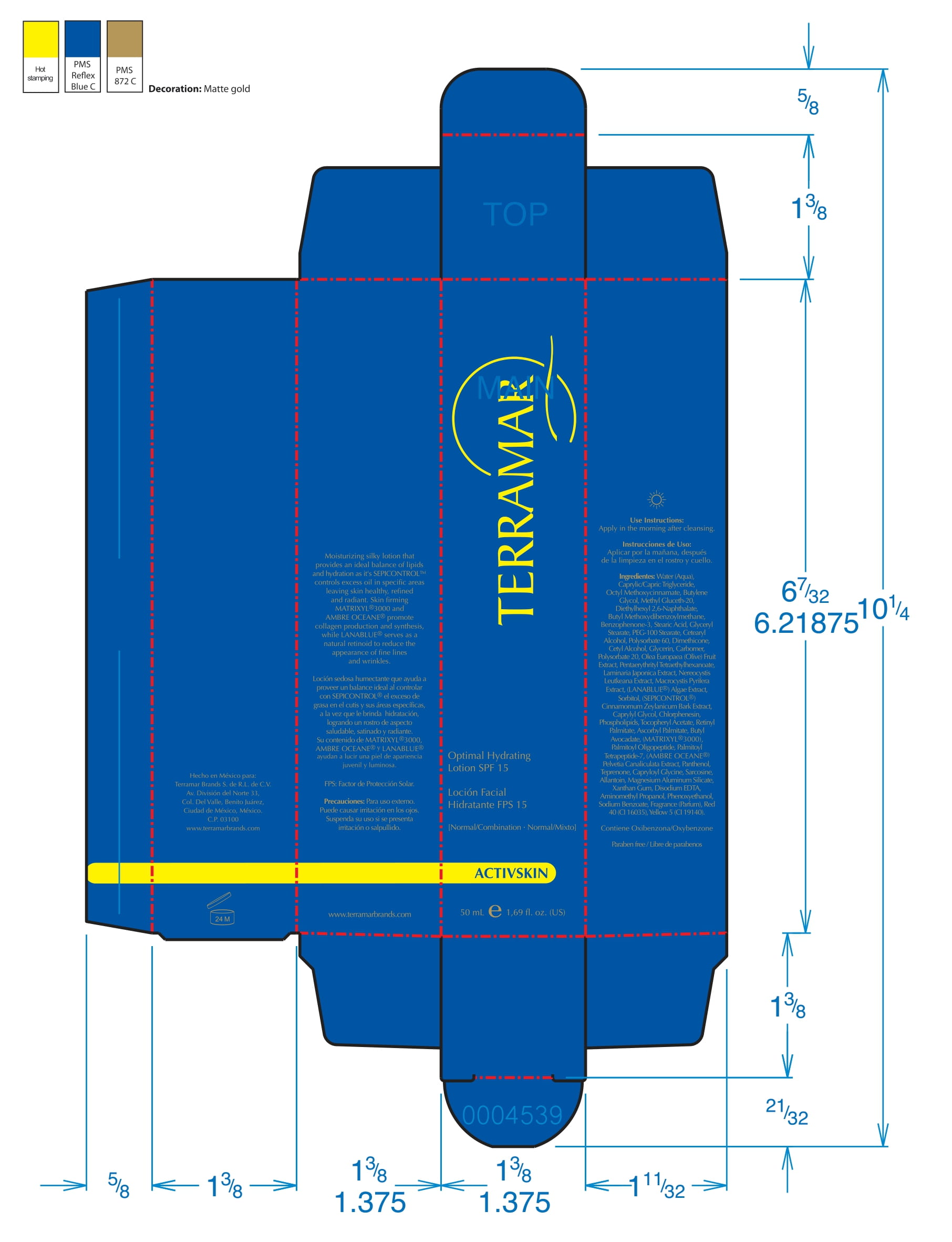
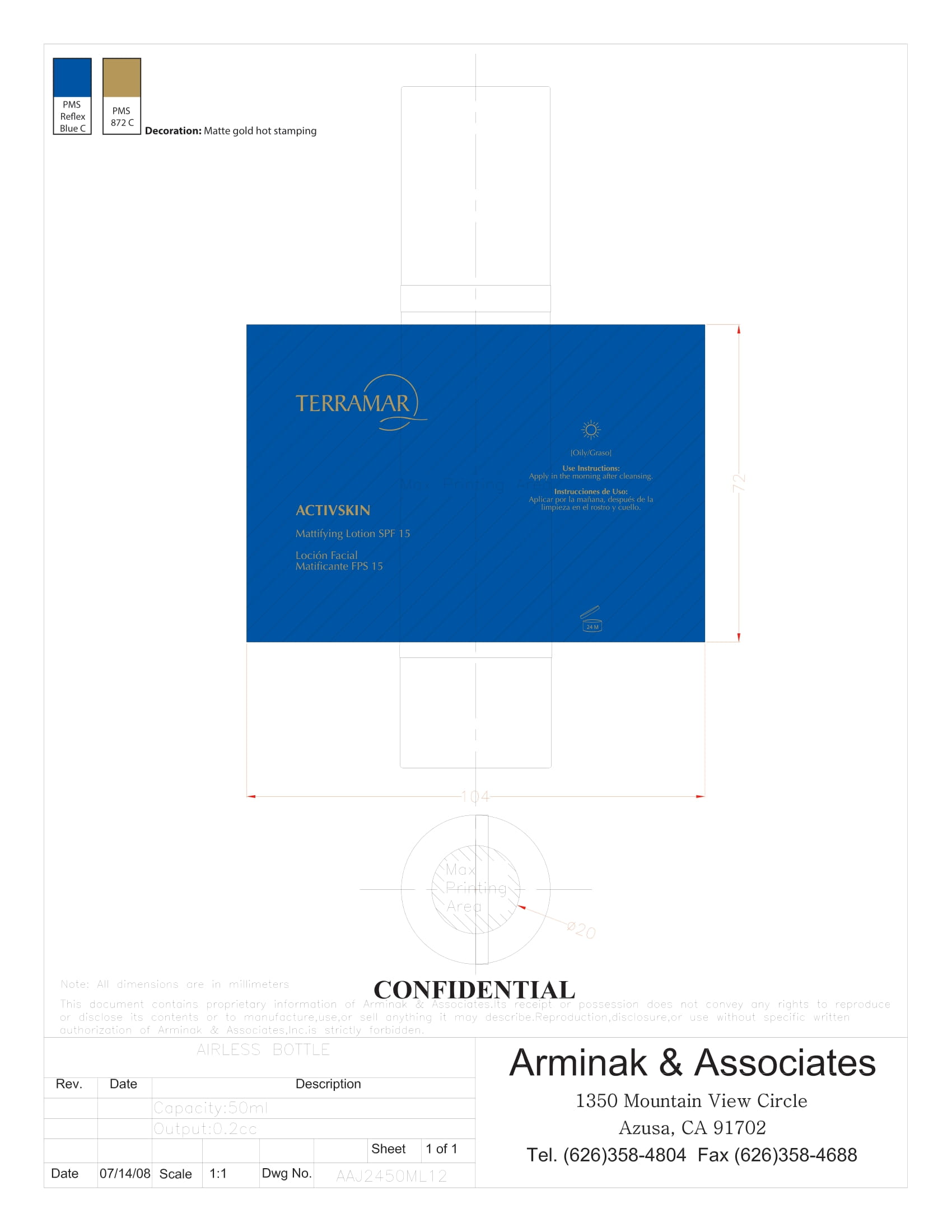
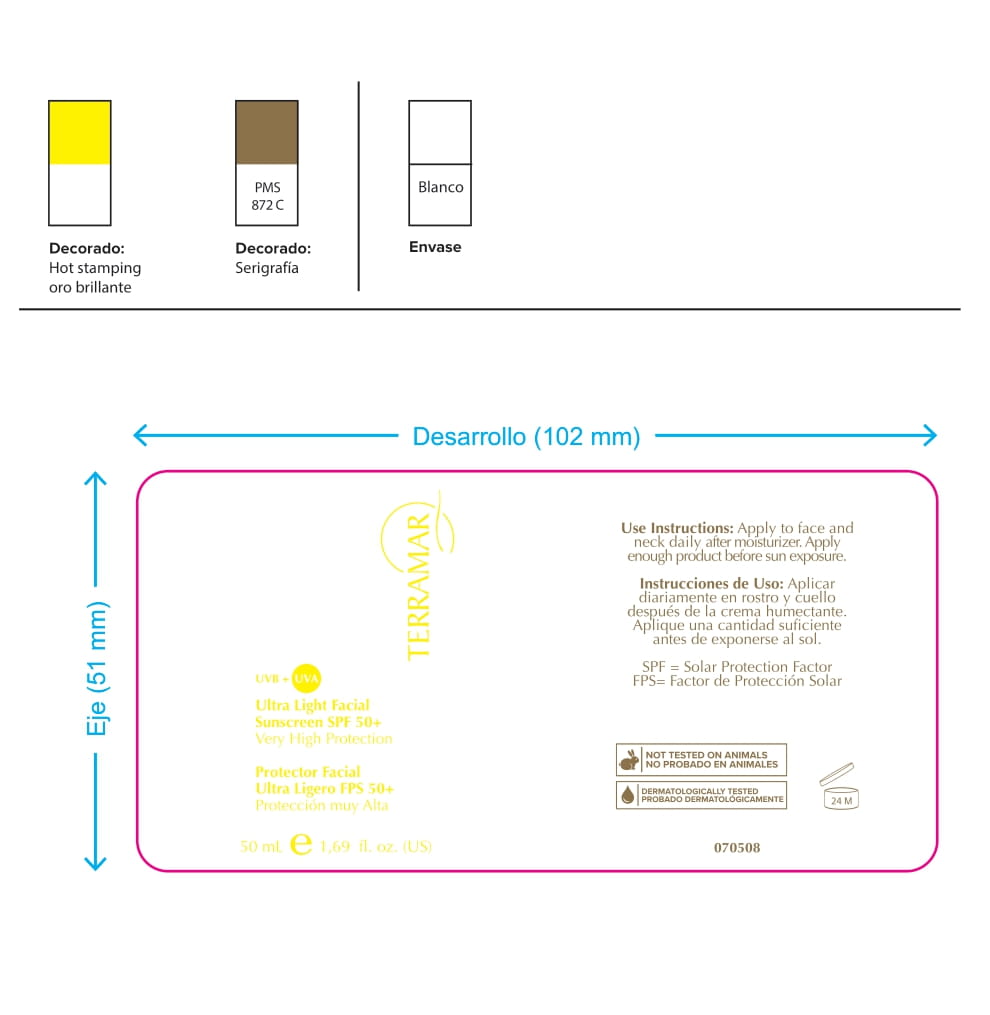
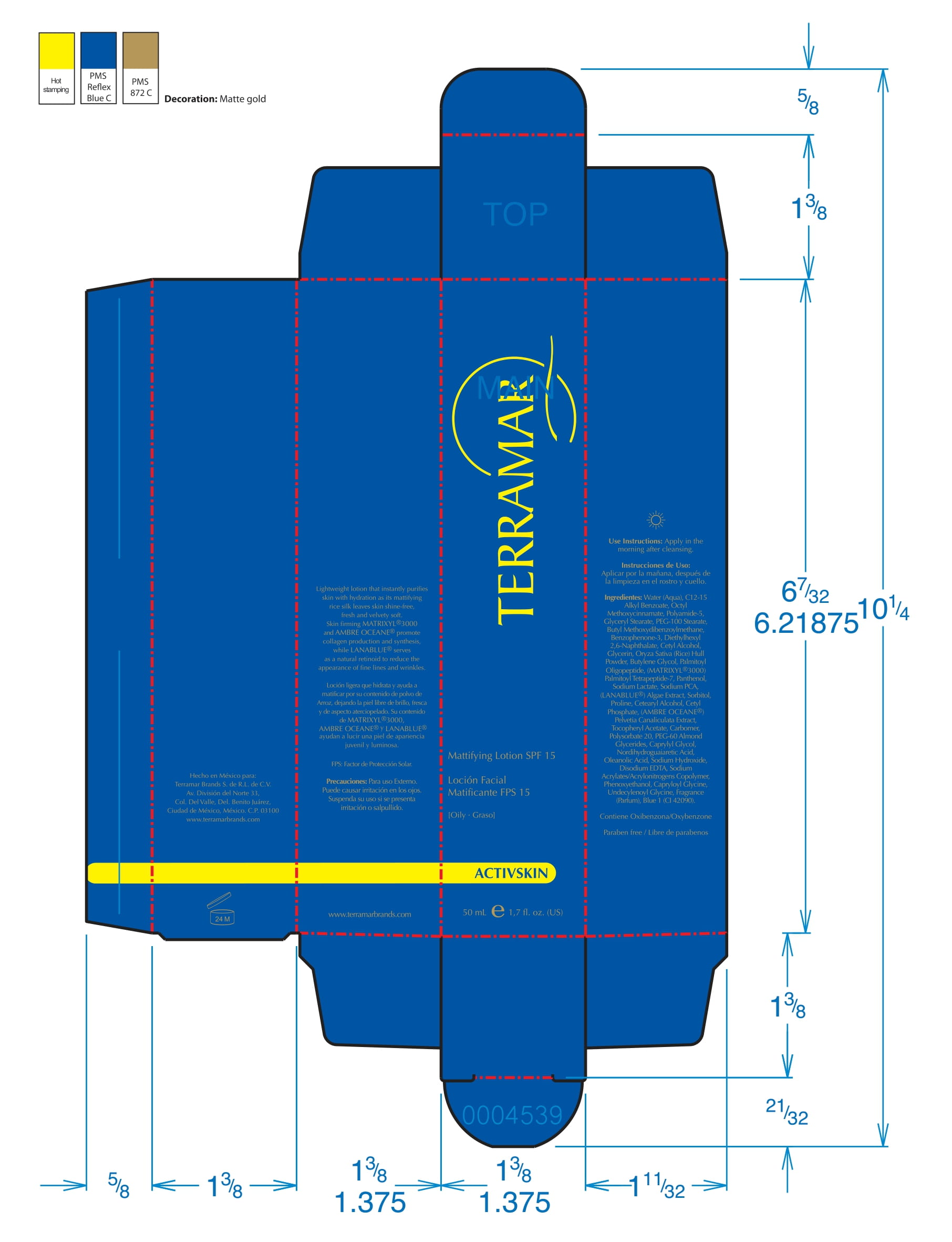
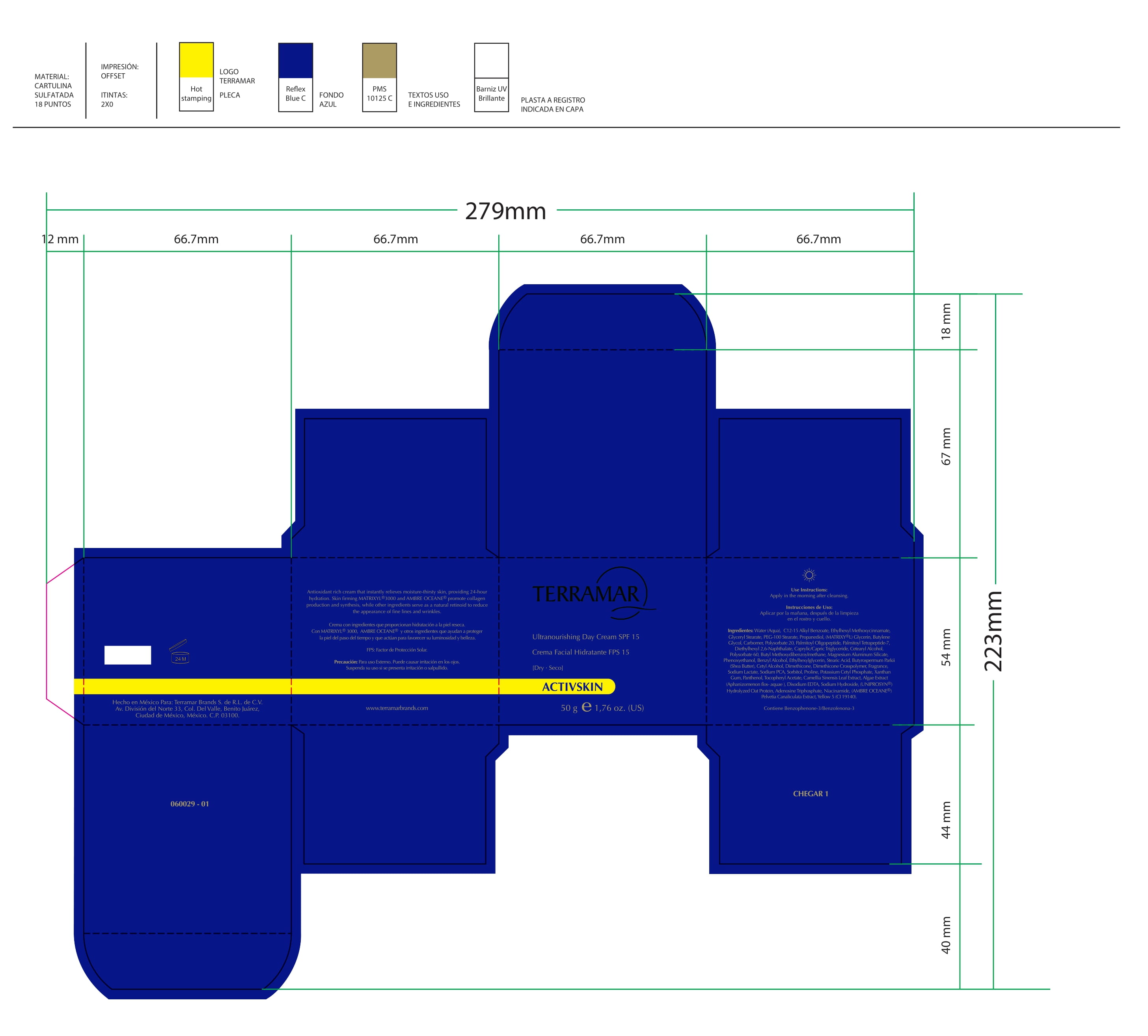
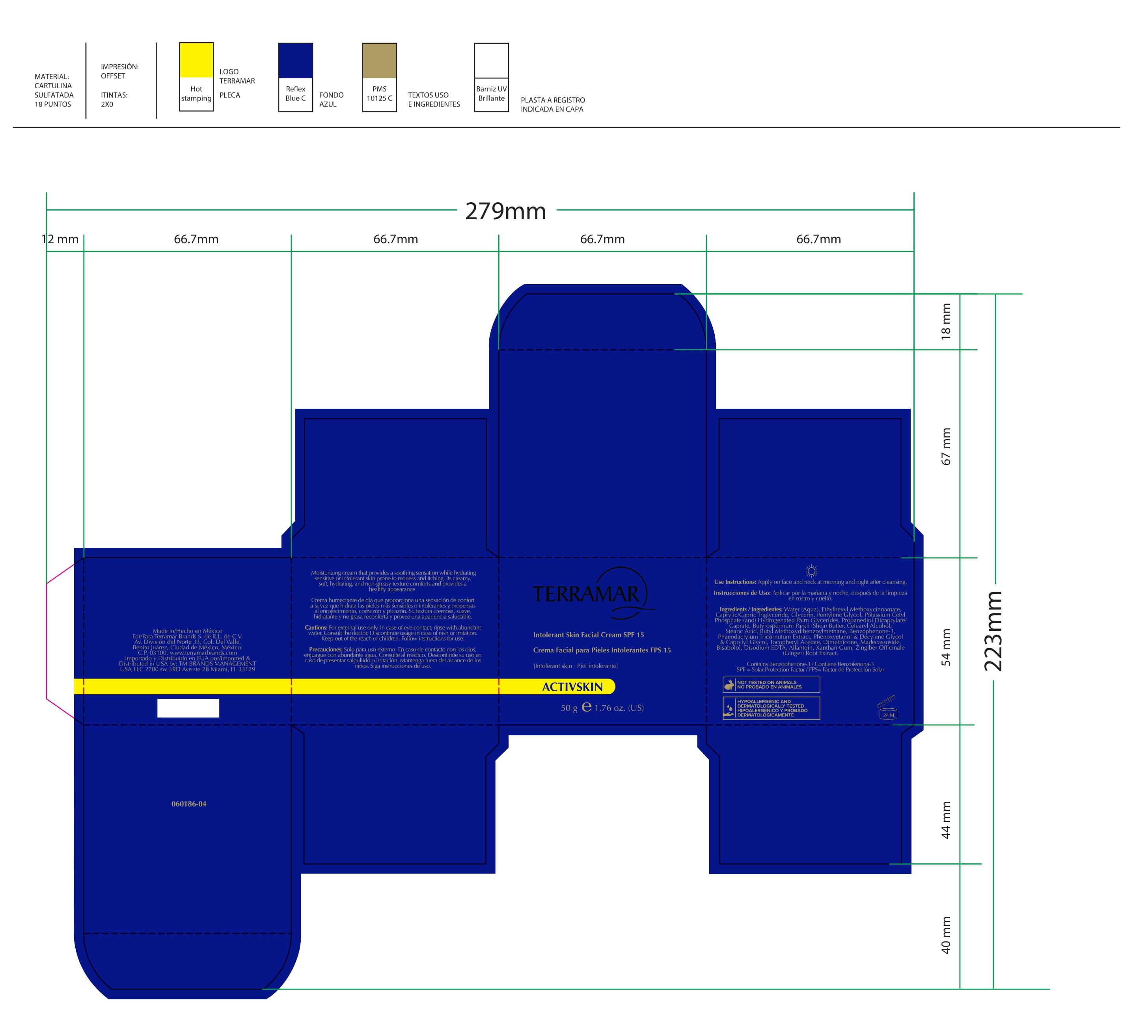
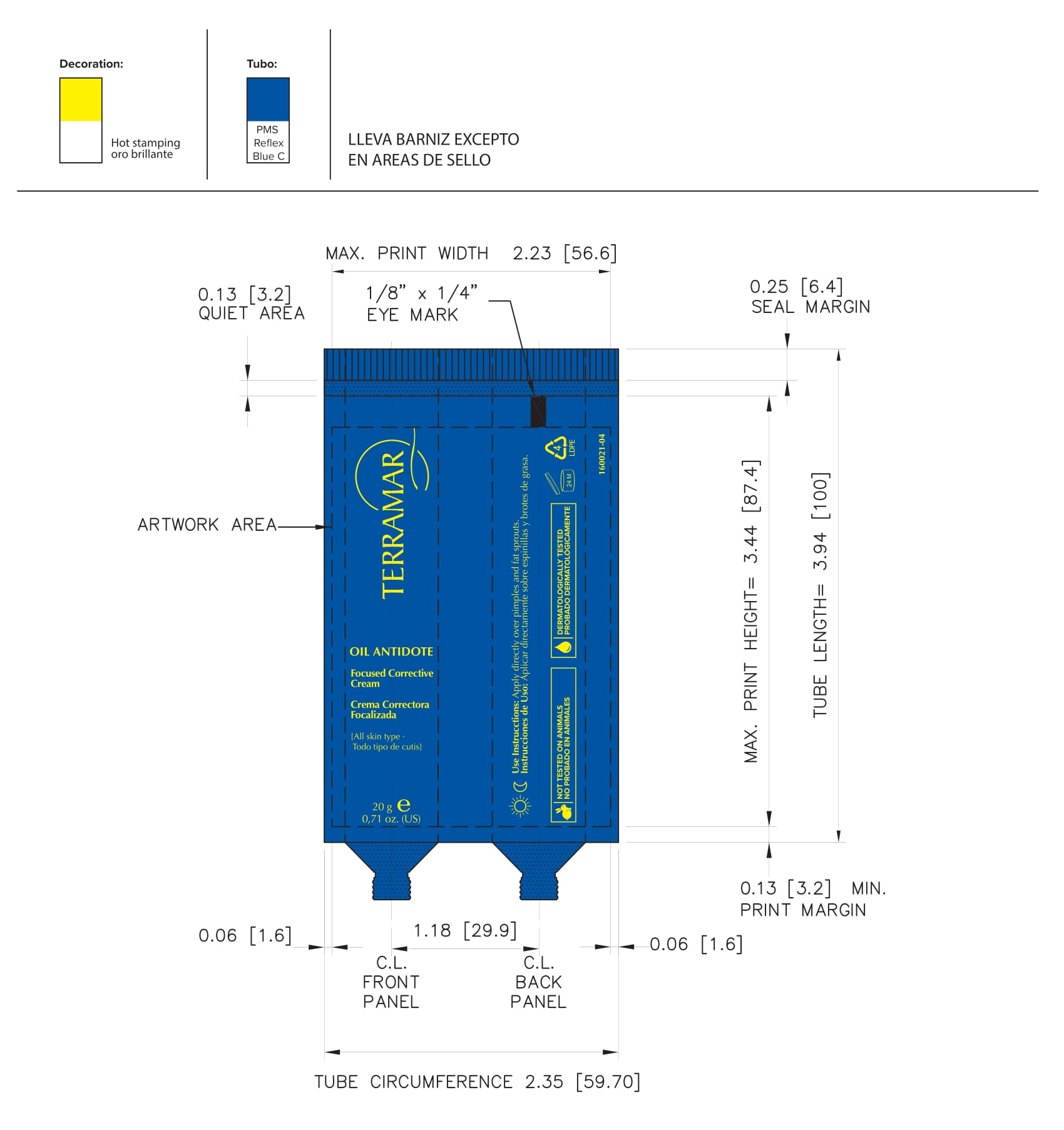
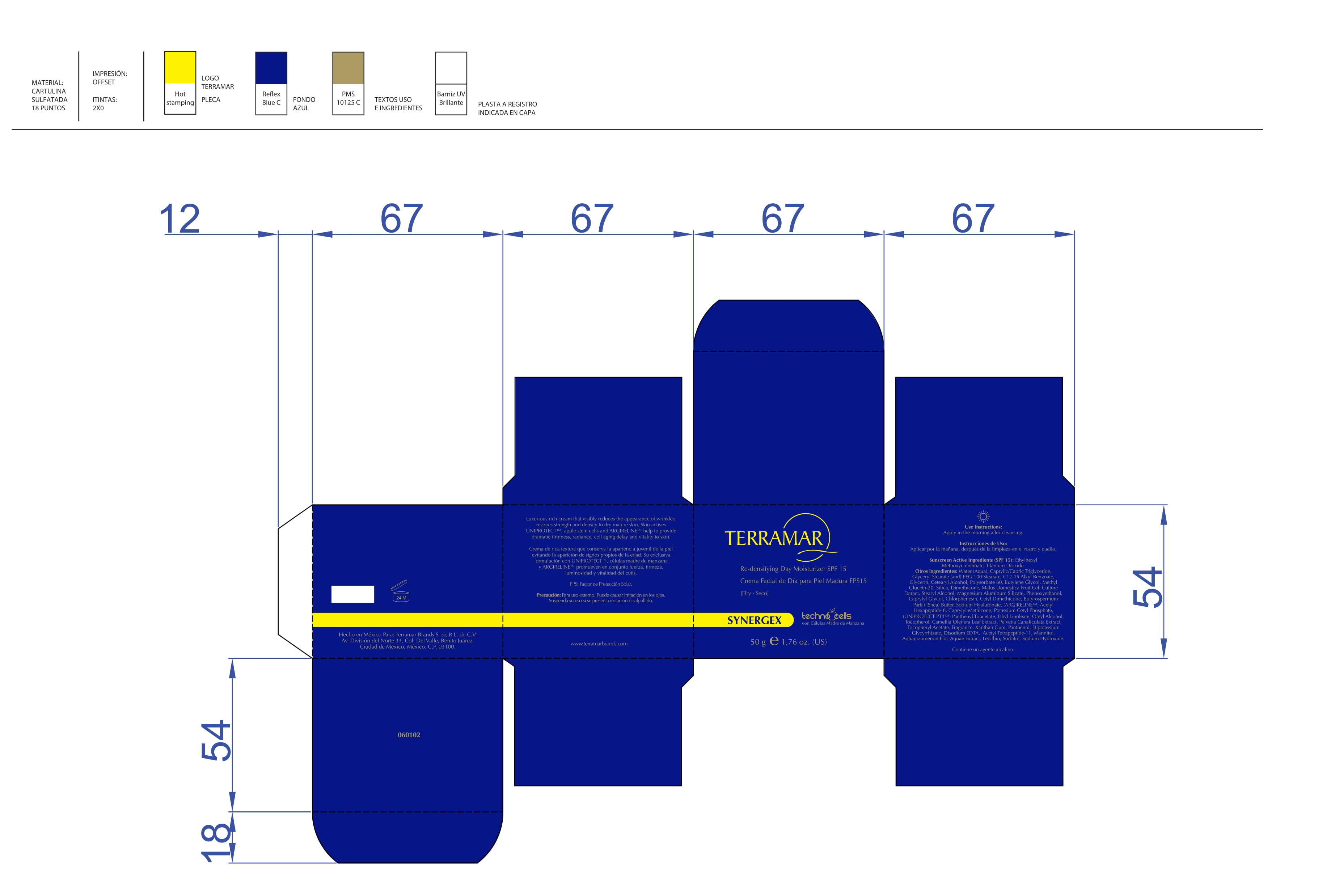
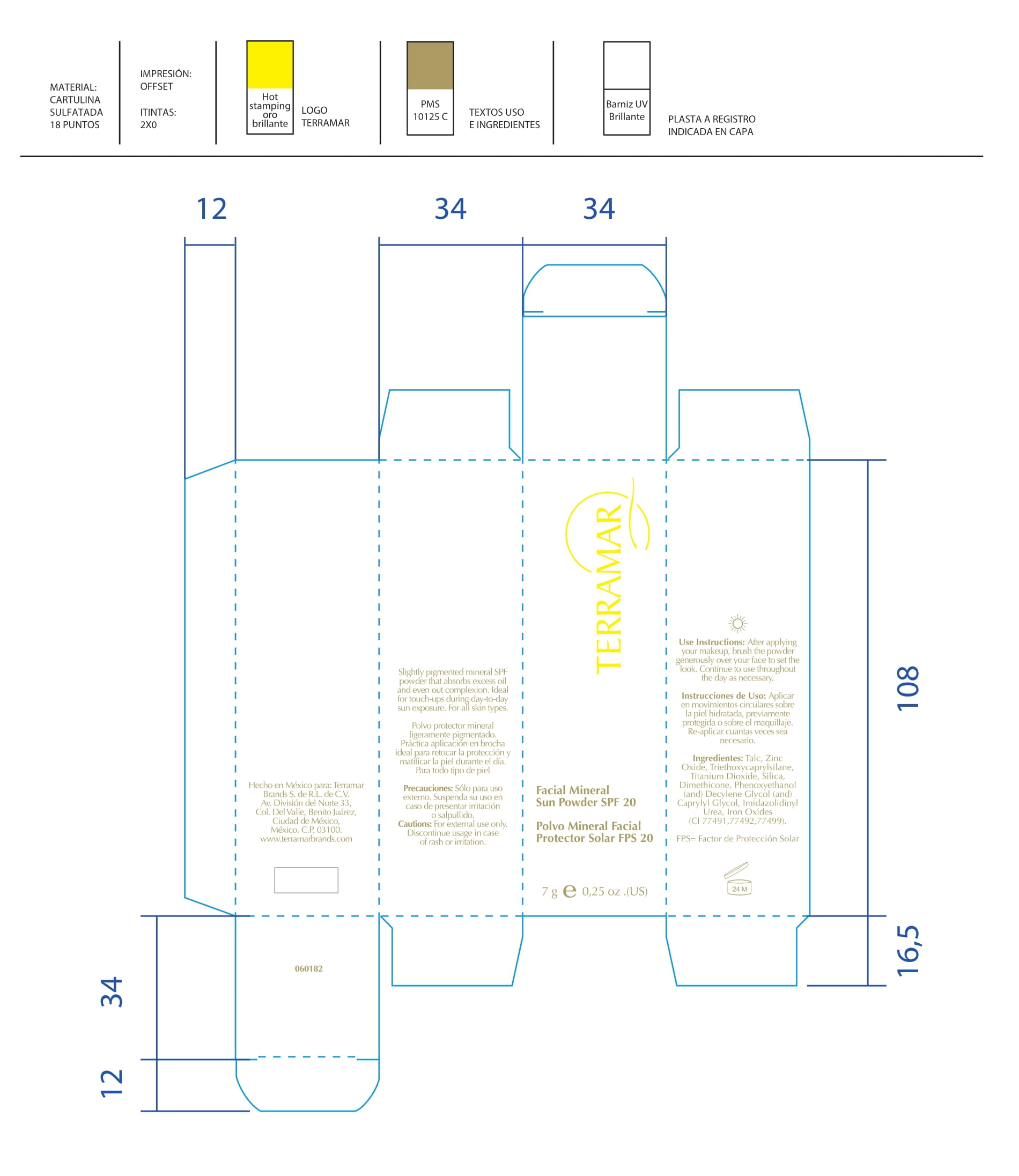
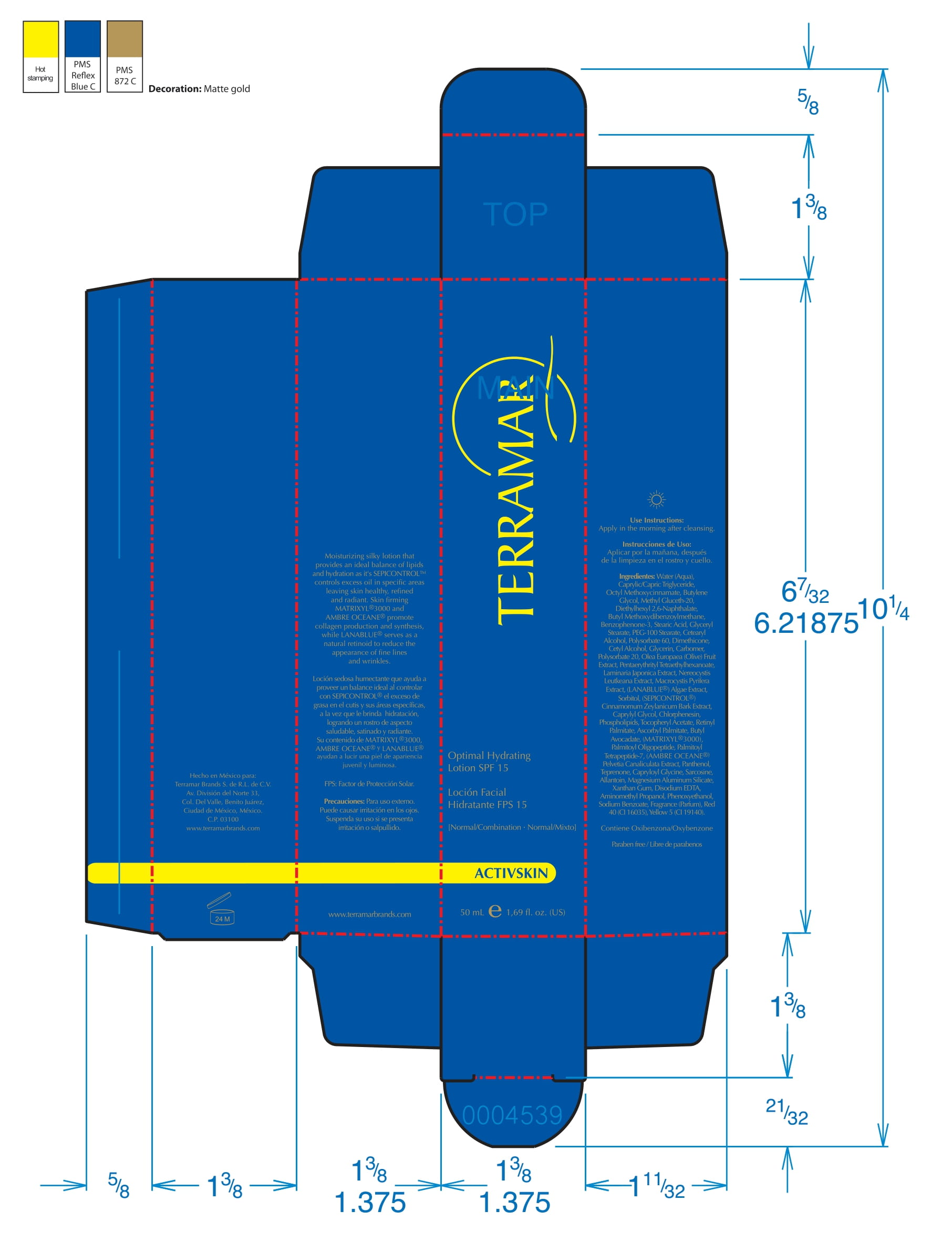
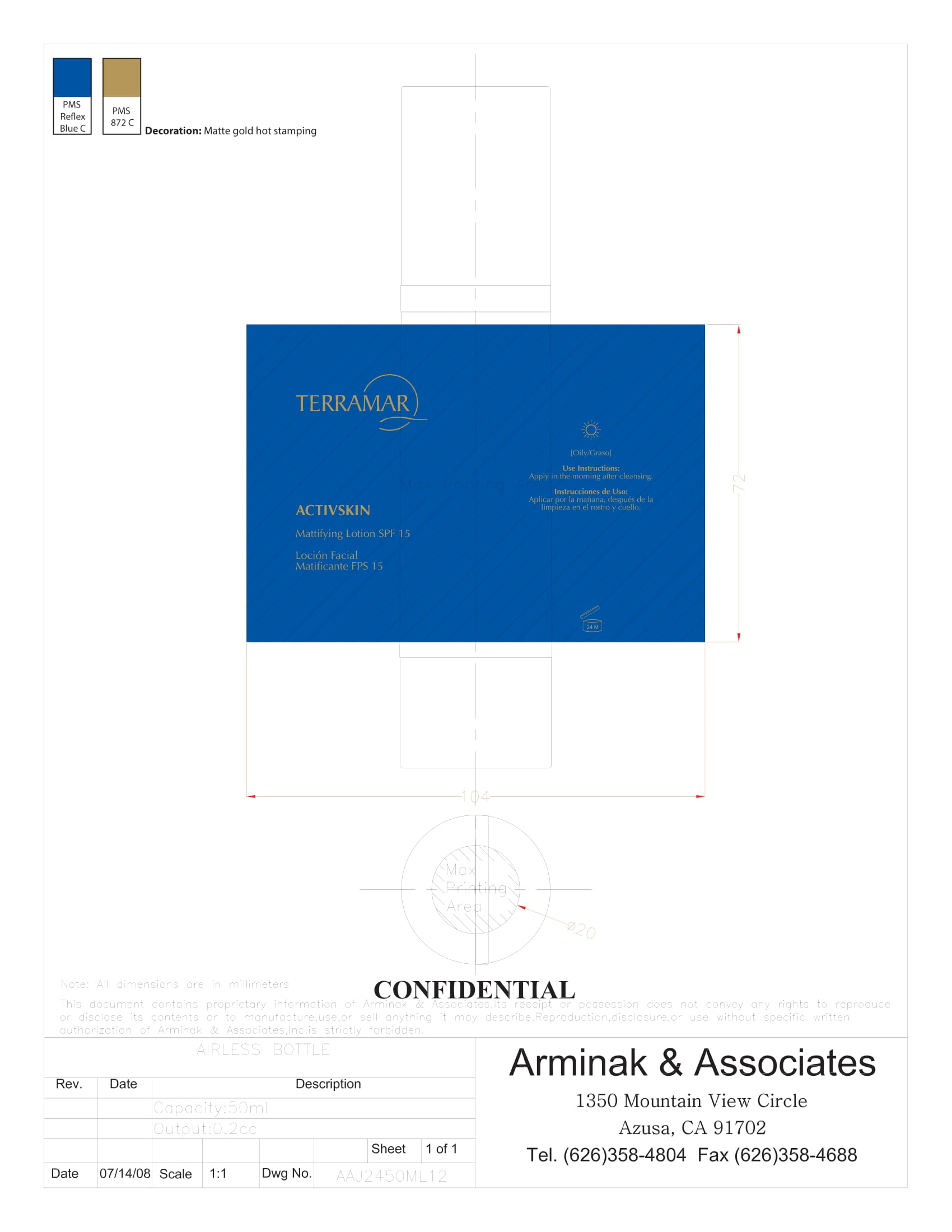
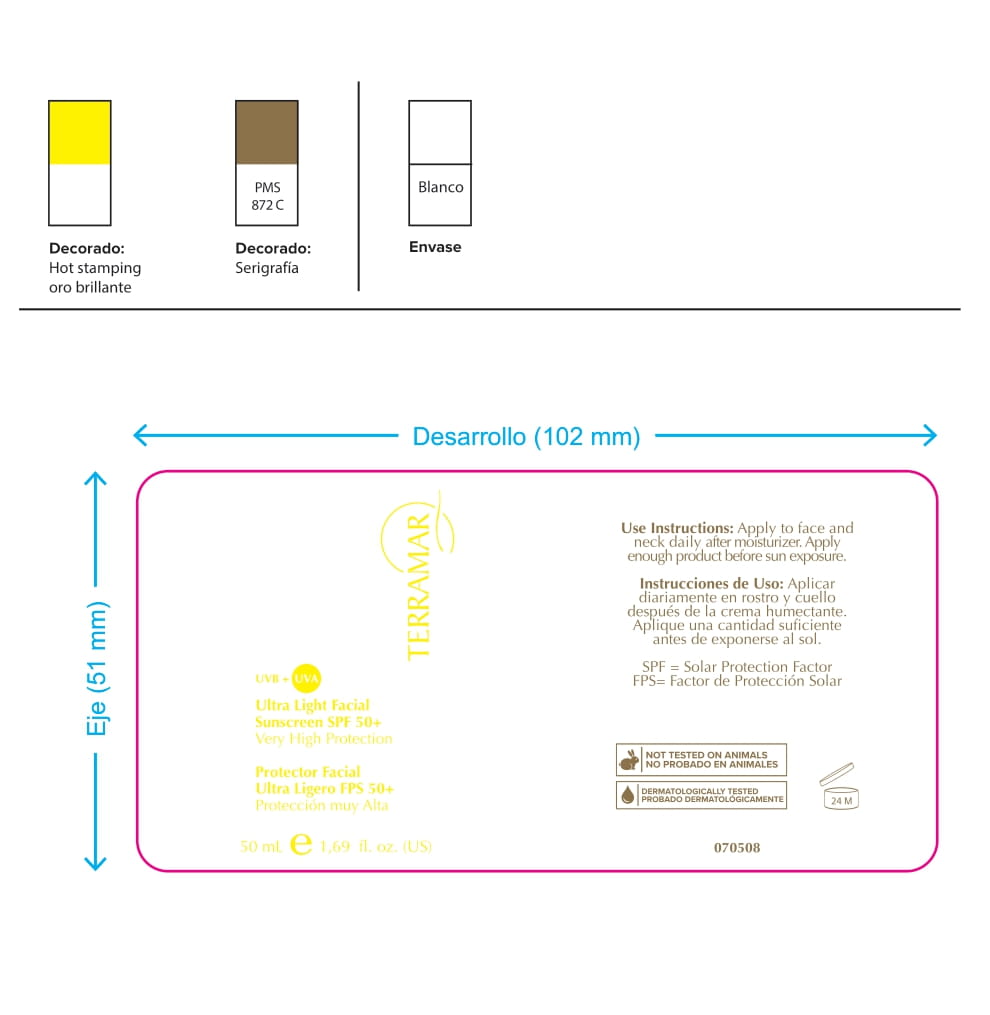
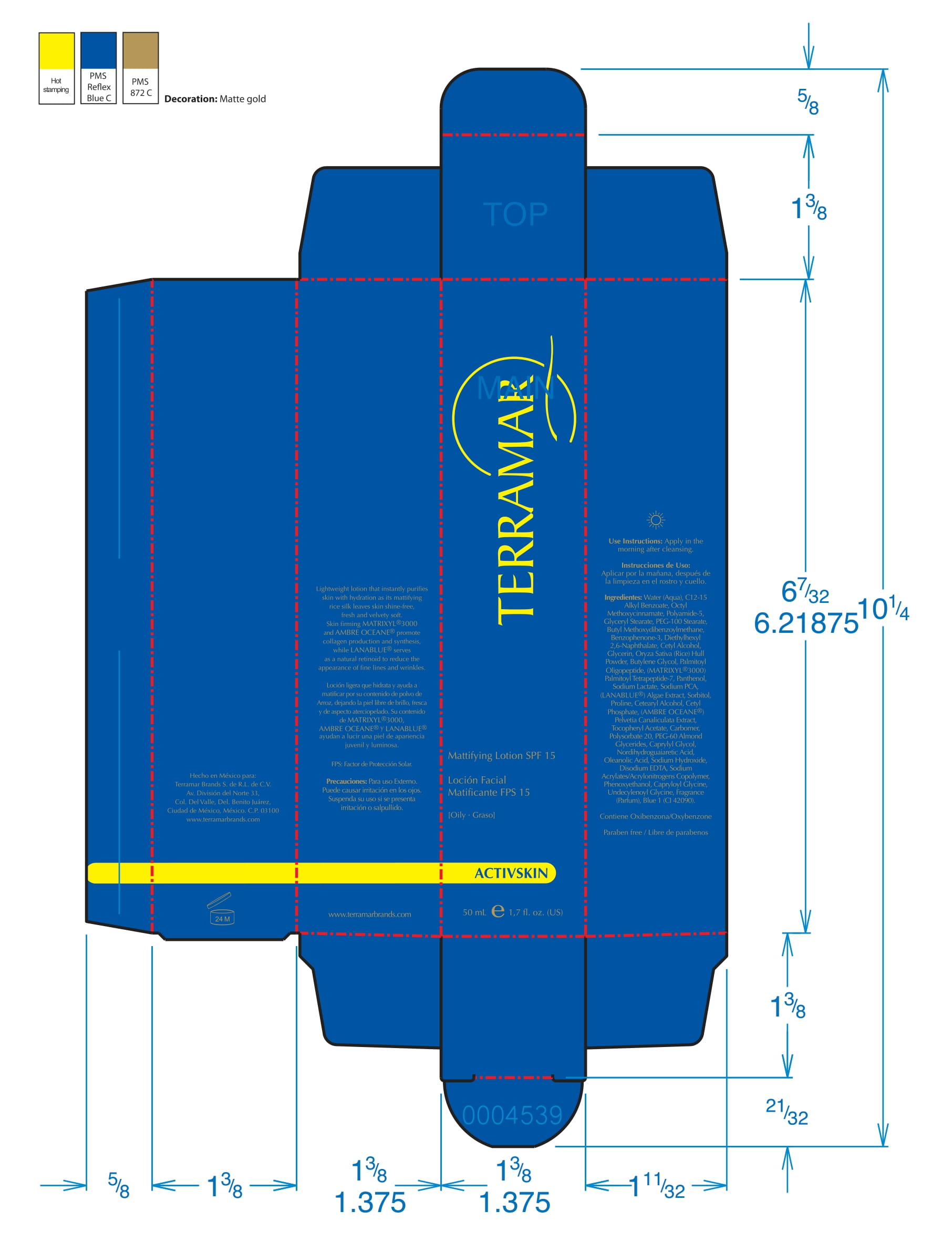
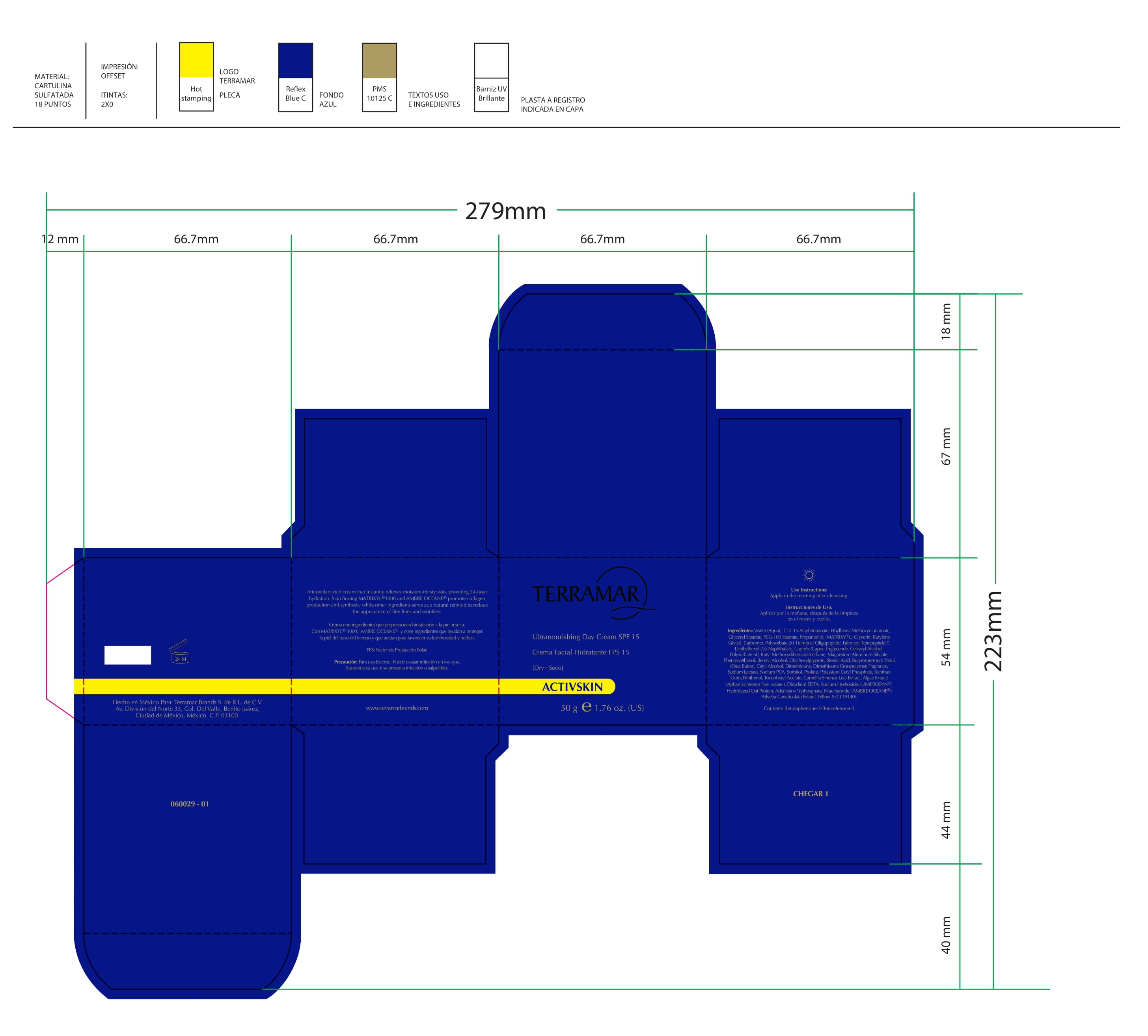
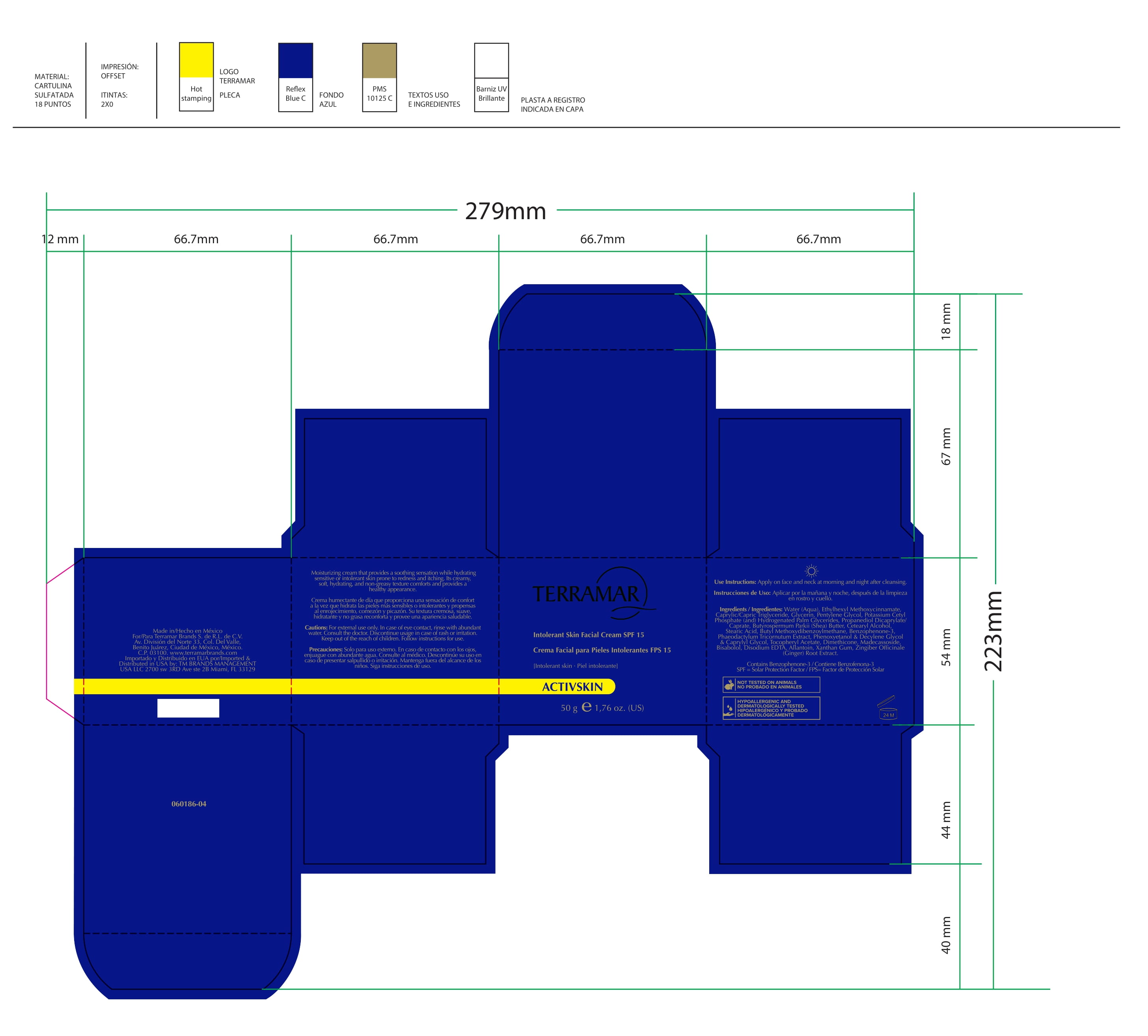
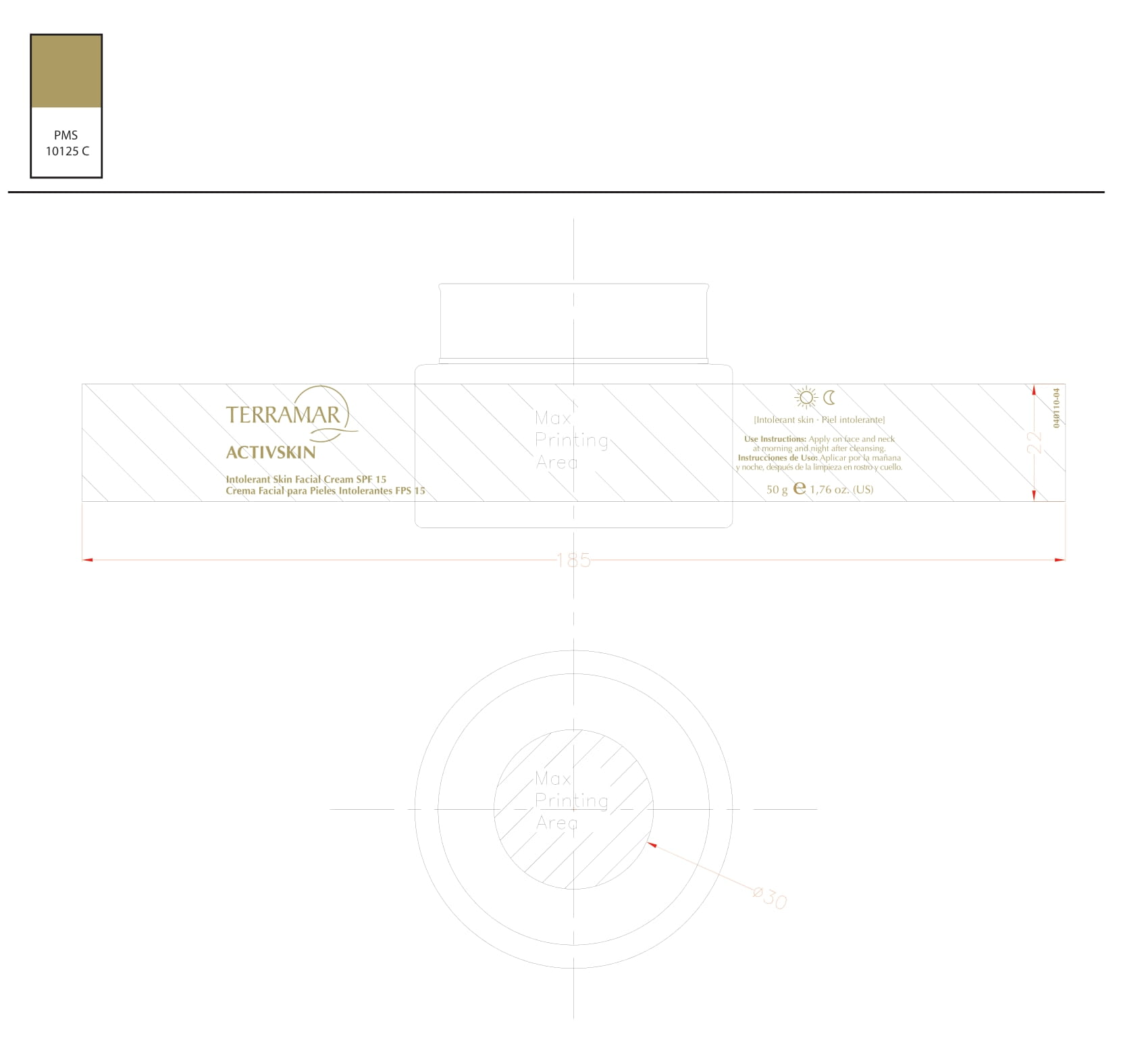
Label: HYDRATING FACIAL CREAM SPF 15 DRY SKIN- ethylhexyl methoxycinnamate, butyl methoxydibenzoylmethane, benzophenone3 cream
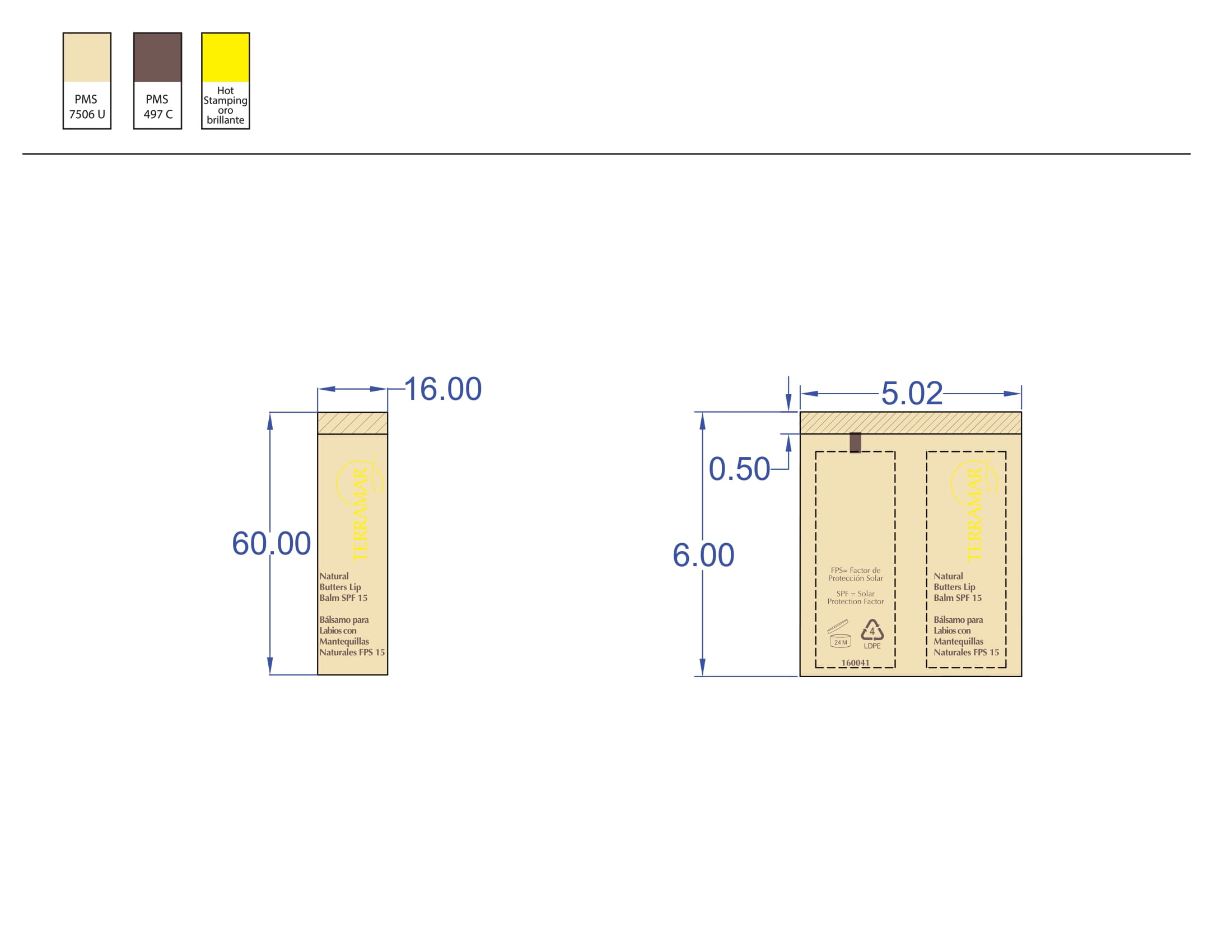
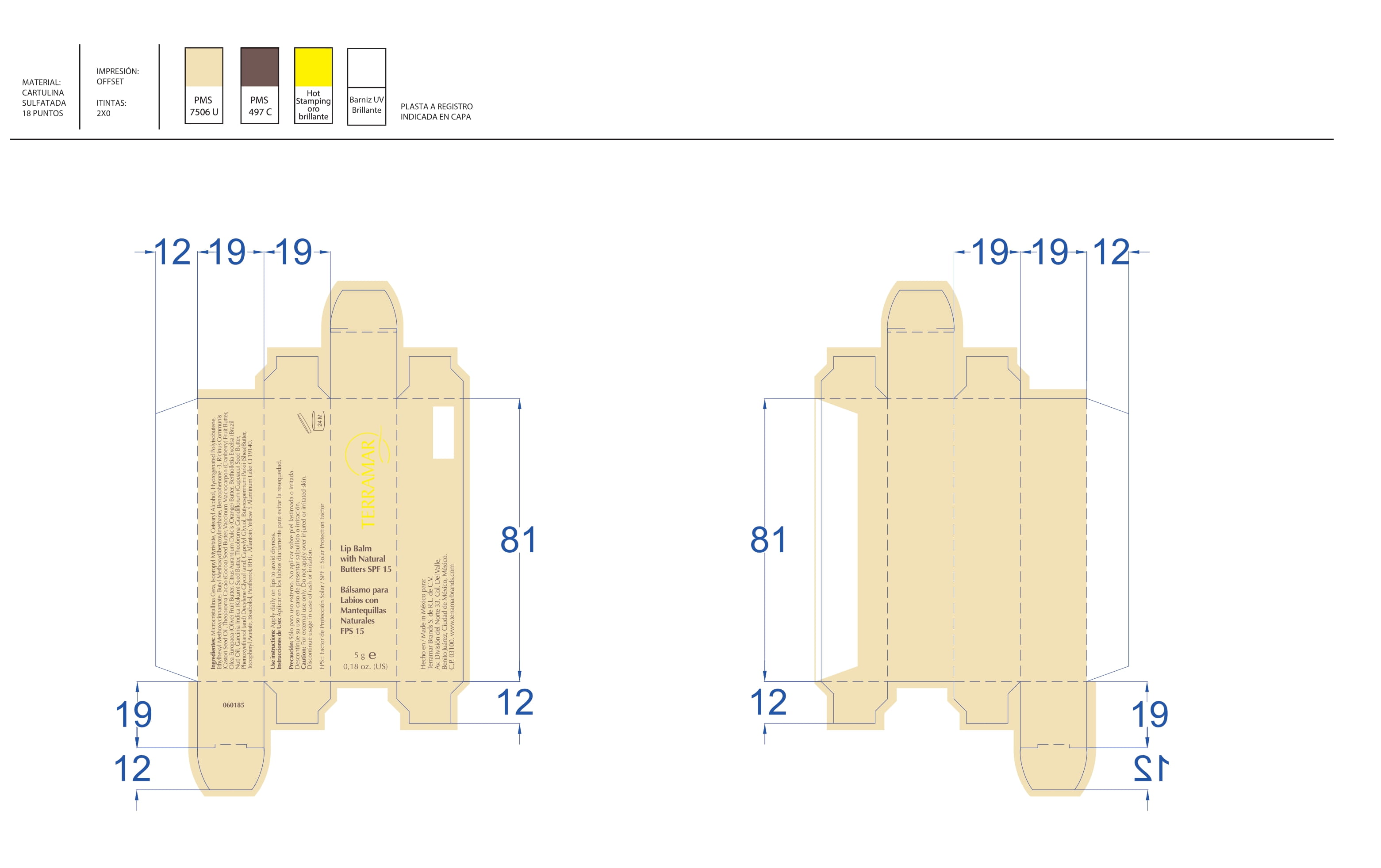
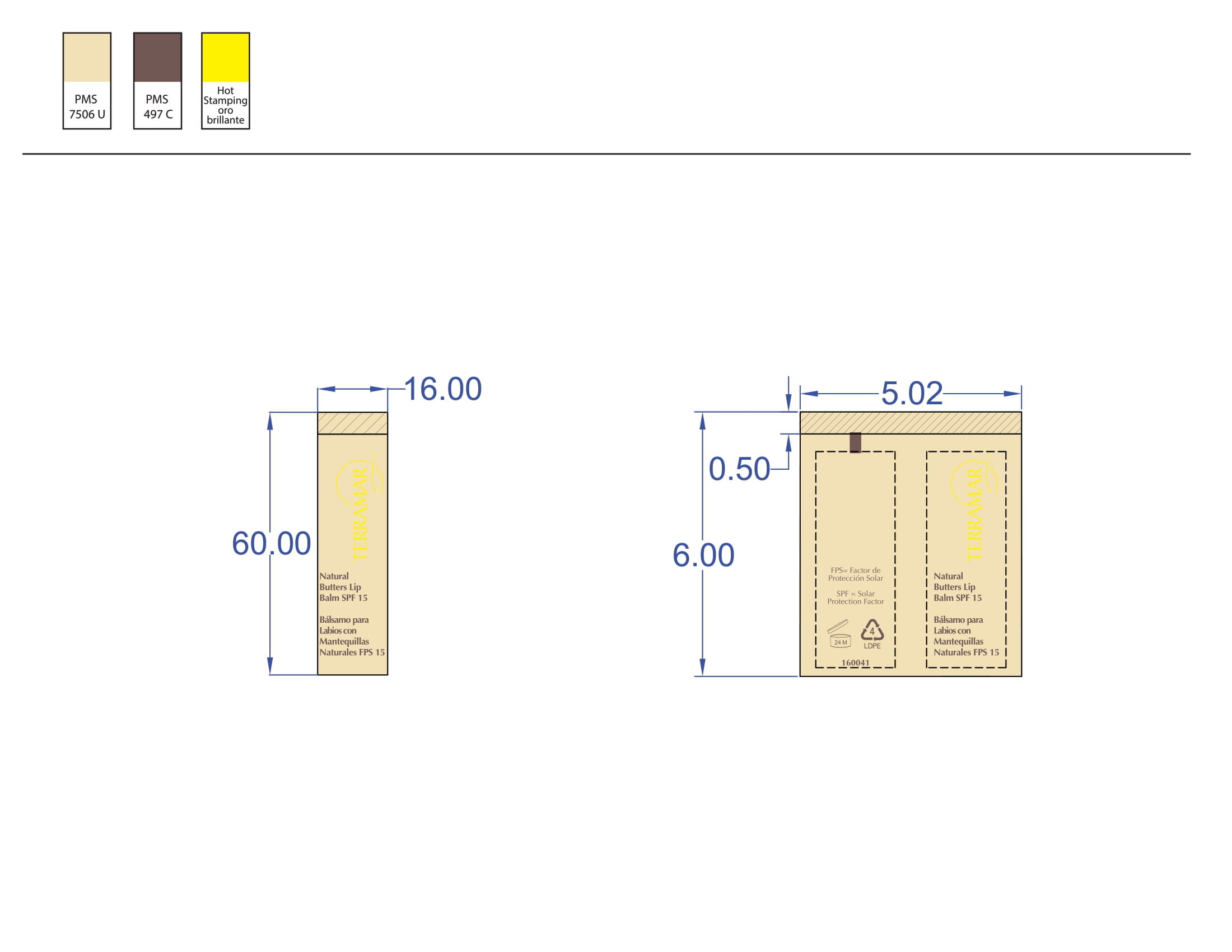
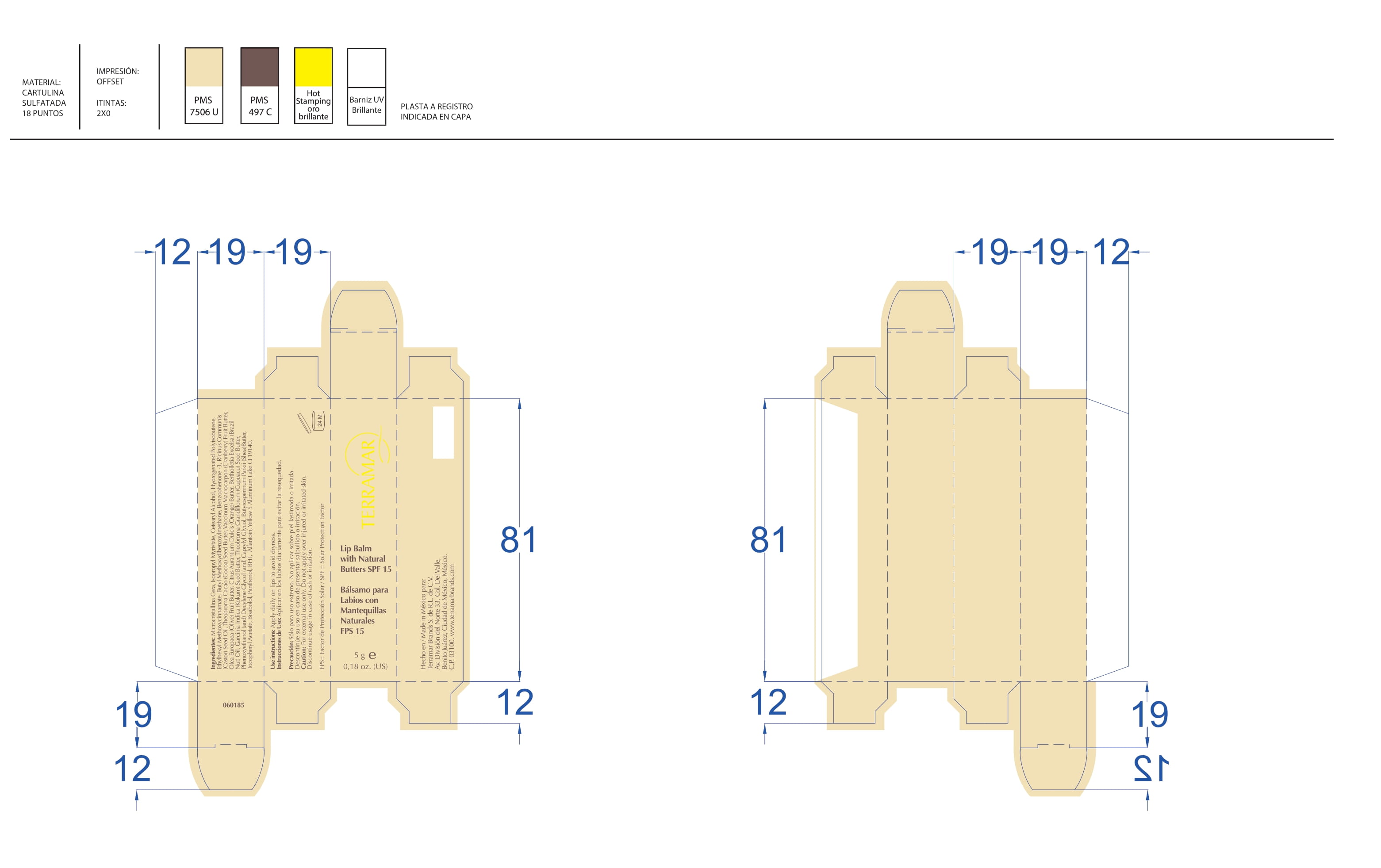
BALM WITH NATURAL BUTTERS SPF15- octinoxate lipstick
EYELASH AND EYEBROW LENGTHENER- propylene glycol cream
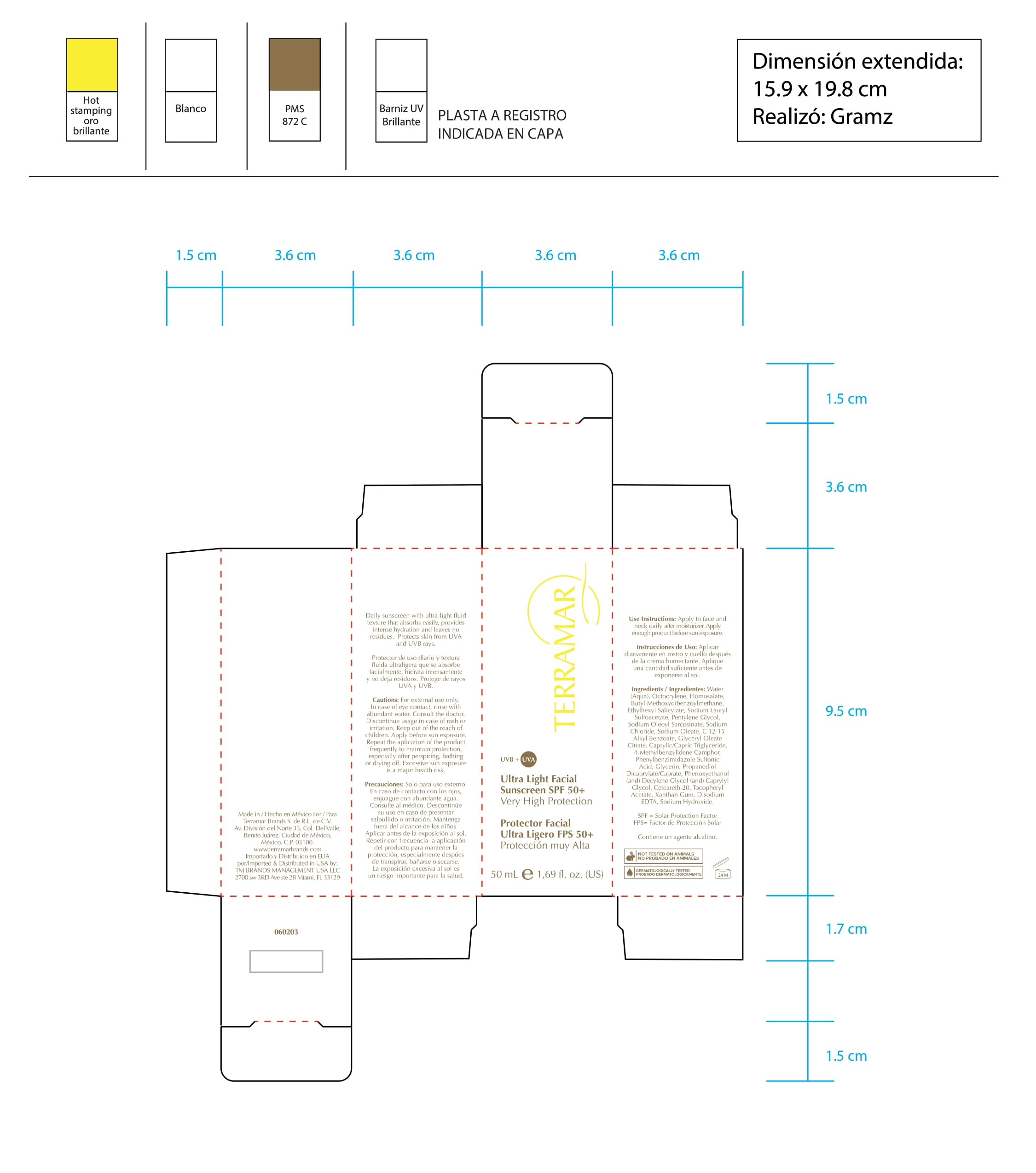
FACIAL PRIMER PROTECTOR SPF 50- homosalate cream
HYDRATING FACIAL SPF 15 MIX NORMAL SKIN .......ray
FACIAL PRIMER PROTECTOR SPF 50- homosalate, benzophenone-3, octocrylene, ethylexyl salicylate, butyl methoxydibenzoylmethane cream
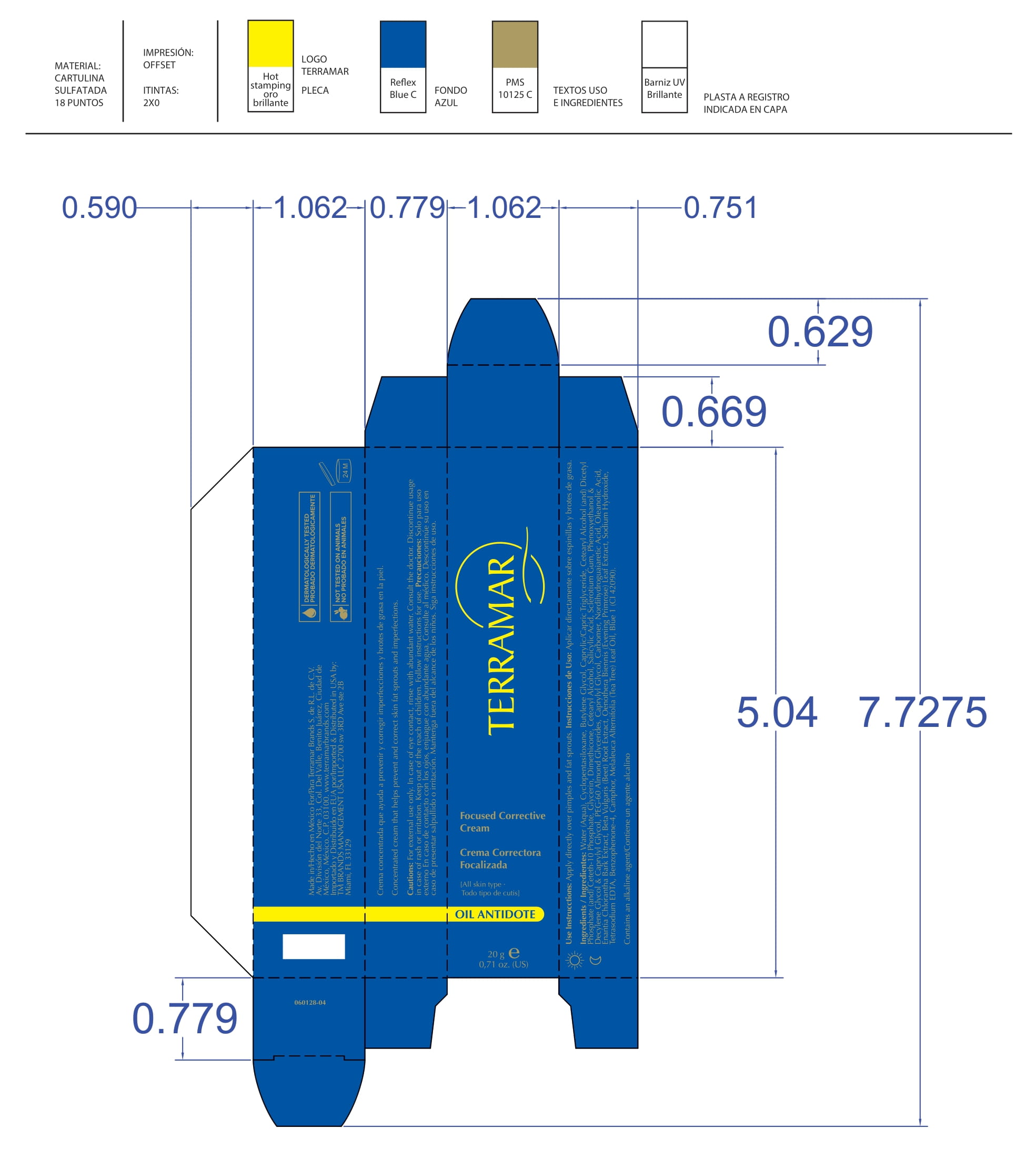
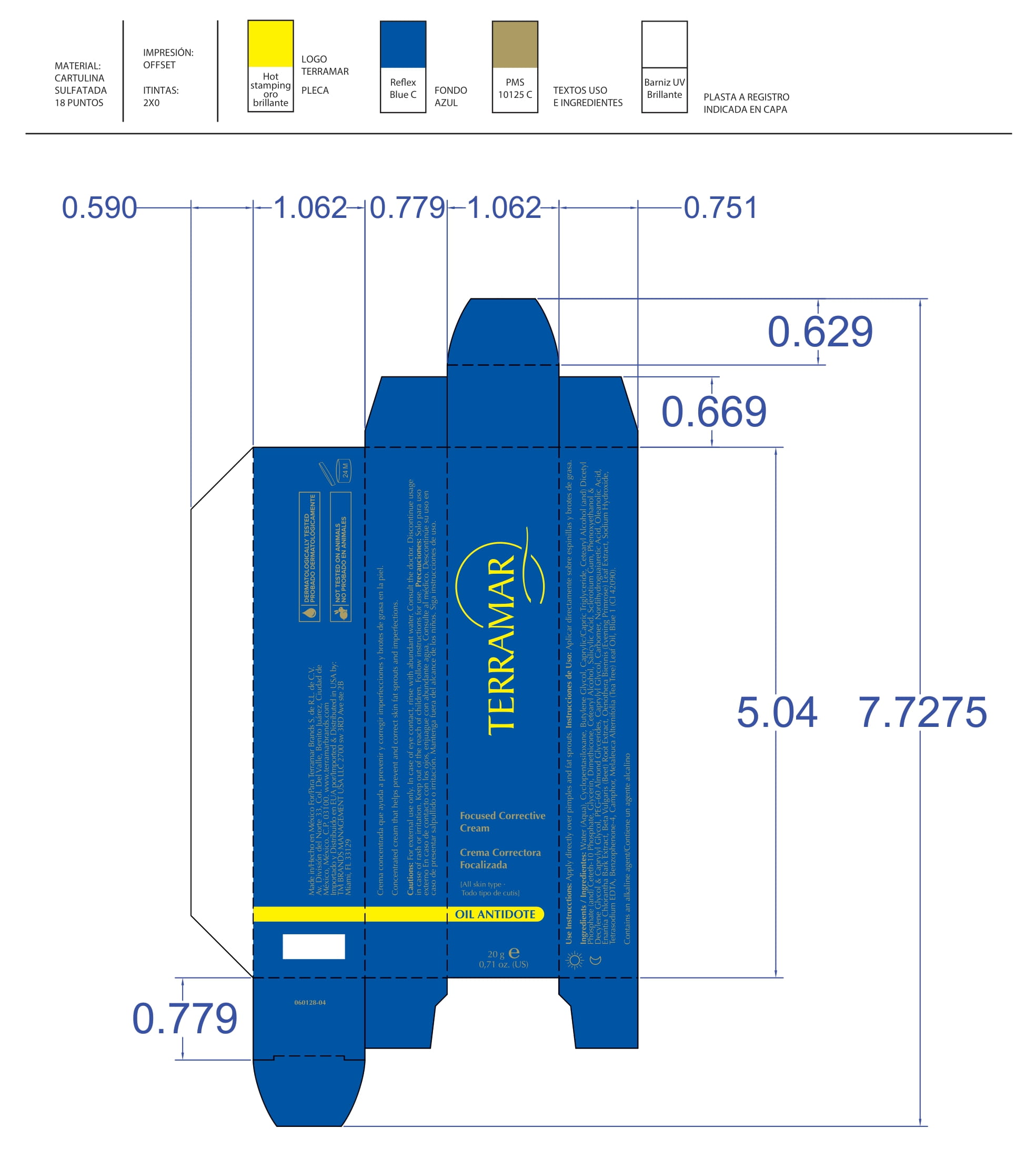
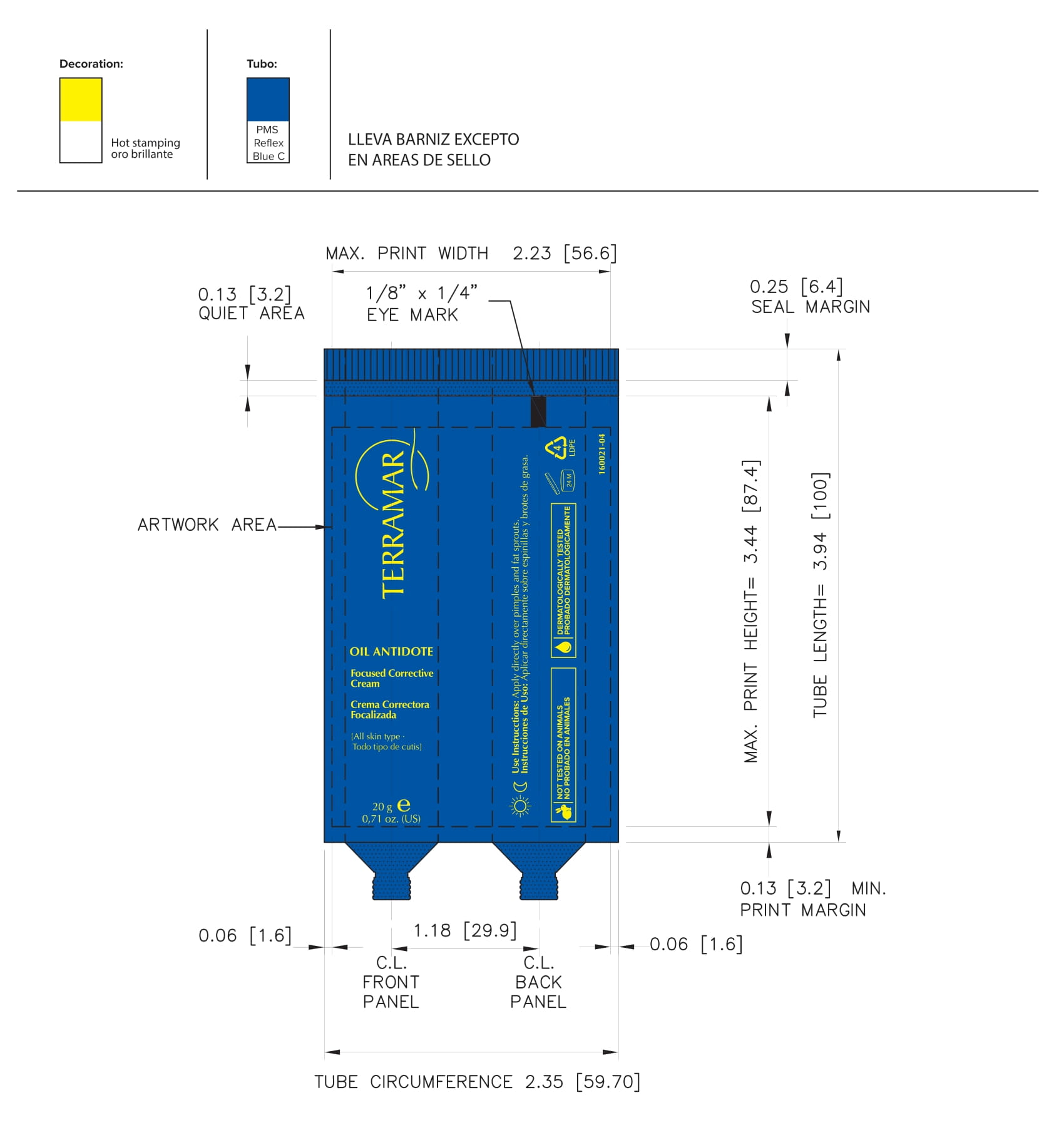
FOCUSED CORRECTIVE (water (aqua), butylene glycol, peg-60 almond glycerides, caprylyl glycol, glycerin, carbomer, nordihydroguaiaretic acid, oleanolic acid, beta vulgaris (beet) root extract, oenothera biennis- evening primrose leaf extract cream
-
Contains inactivated NDC Code(s)
NDC Code(s): 82736-001-01, 82736-002-02, 82736-003-02, 82736-004-01, view more82736-005-01, 82736-006-01, 82736-007-01, 82736-008-01, 82736-009-01, 82736-010-01, 82736-011-01, 82736-012-01, 82736-013-01, 82736-014-01, 82736-015-01, 82736-016-01, 82736-017-01, 82736-018-01, 82736-019-02, 82736-020-01 - Packager: TERRAMAR BRANDS S de RL de CV
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated May 25, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Medication Guide: HTML
- Official Label (Printer Friendly)
- 51945-4
- 51945-4
- 51945-4
- 51945-4
- DOSAGE & ADMINISTRATION
- WARNINGS AND PRECAUTIONS
- INACTIVE INGREDIENT
- INDICATIONS & USAGE
- WARNINGS
- 51945-4
- 51945-4
-
INGREDIENTS AND APPEARANCE
HYDRATING FACIAL CREAM SPF 15 DRY SKIN
ethylhexyl methoxycinnamate, butyl methoxydibenzoylmethane, benzophenone3 creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:82736-008 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 0.1 g in 1 g Inactive Ingredients Ingredient Name Strength HYDRATED SILICA (UNII: Y6O7T4G8P9) 0.7 g in 1 g DIMETHICONE (UNII: 92RU3N3Y1O) 0.1 g in 1 g TITANIUM DIOXIDE (UNII: 15FIX9V2JP) 0.1 g in 1 g Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:82736-008-01 1 g in 1 CARTON; Type 0: Not a Combination Product 05/26/2022 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part352 05/26/2022 BALM WITH NATURAL BUTTERS SPF15
octinoxate lipstickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:82736-010 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 0.1 g in 1 g Inactive Ingredients Ingredient Name Strength AVOBENZONE (UNII: G63QQF2NOX) 0.8 g in 1 g BENZOPHENONE-2 (UNII: PRR8K3H9VN) 0.1 g in 1 g Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:82736-010-01 1 g in 1 CARTON; Type 0: Not a Combination Product 05/26/2022 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 05/26/2022 EYELASH AND EYEBROW LENGTHENER
propylene glycol creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:82736-019 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PROPYLENE GLYCOL (UNII: 6DC9Q167V3) (PROPYLENE GLYCOL - UNII:6DC9Q167V3) PROPYLENE GLYCOL 0.1 g in 1 g Inactive Ingredients Ingredient Name Strength MYRISTOYL PENTAPEPTIDE-4 (UNII: PMA59A699X) 0.1 g in 1 g WATER (UNII: 059QF0KO0R) 0.7 g in 1 g GLYCERIN (UNII: PDC6A3C0OX) 0.1 g in 1 g Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:82736-019-02 1 g in 1 CARTON; Type 0: Not a Combination Product 05/26/2022 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part349 05/26/2022 FACIAL PRIMER PROTECTOR SPF 50
homosalate creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:82736-020 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 0.1 g in 1 g Inactive Ingredients Ingredient Name Strength BENZOPHENONE-2 (UNII: PRR8K3H9VN) 0.7 g in 1 g BUTYL OXOACETATE (UNII: AH46A7531R) 0.1 g in 1 g OCTOCRYLENE (UNII: 5A68WGF6WM) 0.1 g in 1 g Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:82736-020-01 1 g in 1 CARTON; Type 0: Not a Combination Product 05/26/2022 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 05/26/2022 HYDRATING FACIAL SPF 15 MIX NORMAL SKIN
octinoxate creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:82736-012 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 0.1 g in 1 g Inactive Ingredients Ingredient Name Strength OXYBENZONE (UNII: 95OOS7VE0Y) 0.8 g in 1 g AVOBENZONE (UNII: G63QQF2NOX) 0.1 g in 1 g Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:82736-012-01 1 g in 1 CARTON; Type 0: Not a Combination Product 05/26/2022 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 05/26/2022 ULTRA LIGHT FACIAL SUNSCREEN SPF 50
octocrylene creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:82736-015 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 0.1 g in 1 g AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 0.8 g in 1 g Inactive Ingredients Ingredient Name Strength OXYBENZONE (UNII: 95OOS7VE0Y) 0.1 g in 1 g Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:82736-015-01 1 g in 1 CARTON; Type 0: Not a Combination Product 05/26/2022 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 05/26/2022 ANTI LOSS TREATMENT TERRAMAR
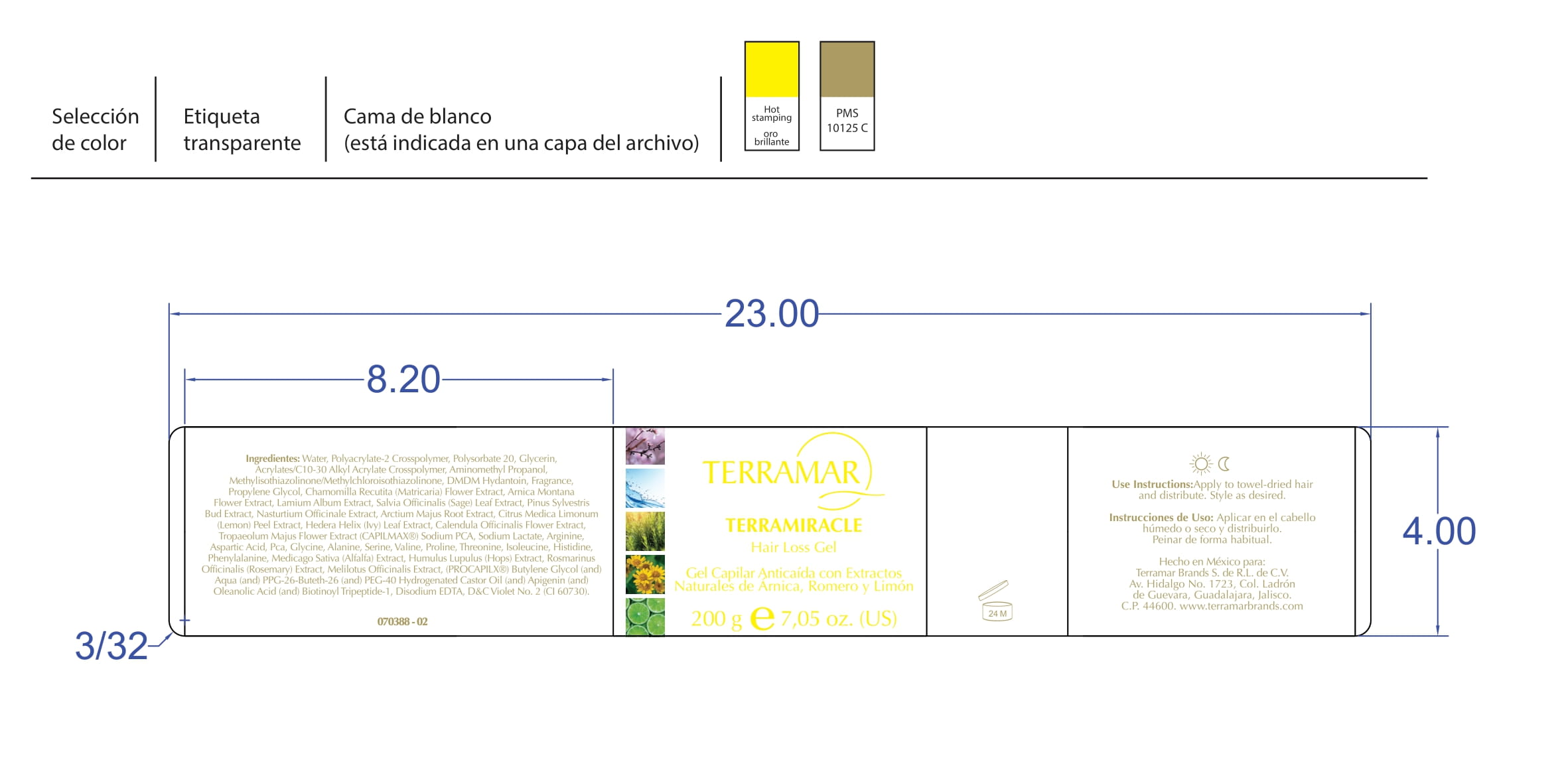
3-hexyloxypropylene glycol creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:82736-018 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength 3-HEXYLOXYPROPYLENE GLYCOL (UNII: 3485P35DA4) (3-HEXYLOXYPROPYLENE GLYCOL - UNII:3485P35DA4) 3-HEXYLOXYPROPYLENE GLYCOL 0.1 g in 1 g SALICYLIC ACID (UNII: O414PZ4LPZ) (SALICYLIC ACID - UNII:O414PZ4LPZ) SALICYLIC ACID 0.1 g in 1 g Inactive Ingredients Ingredient Name Strength ARNICA CORDIFOLIA FLOWER (UNII: JCG1OSZ7A8) 0.1 g in 1 g SALVIA APIANA LEAF (UNII: Z29Z21AHMP) 0.7 g in 1 g Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:82736-018-01 1 g in 1 CARTON; Type 0: Not a Combination Product 05/26/2022 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final M032 05/26/2022 ANTILOSS TERRAMAR SH
propylene glycol 1 - oleate creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:82736-017 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PROPYLENE GLYCOL 1-OLEATE (UNII: 476821E6GS) (PROPYLENE GLYCOL 1-OLEATE - UNII:476821E6GS) PROPYLENE GLYCOL 1-OLEATE 0.1 g in 1 g SALICYLIC ACID (UNII: O414PZ4LPZ) (SALICYLIC ACID - UNII:O414PZ4LPZ) SALICYLIC ACID 0.1 g in 1 g Inactive Ingredients Ingredient Name Strength ARNICA CHAMISSONIS FLOWER (UNII: 88WK5I8R3L) 0.7 g in 1 g SALVIA APIANA LEAF (UNII: Z29Z21AHMP) 0.1 g in 1 g Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:82736-017-01 1 g in 1 CARTON; Type 0: Not a Combination Product 05/26/2022 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final M032 05/26/2022 SKIN RECOVERY FACE SPF 15
octinoxate creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:82736-011 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 0.1 g in 1 g Inactive Ingredients Ingredient Name Strength TITANIUM DIOXIDE (UNII: 15FIX9V2JP) 0.7 g in 1 g DIMETHICONE CROSSPOLYMER (450000 MPA.S AT 12% IN CYCLOPENTASILOXANE) (UNII: UF7620L1W6) 0.1 g in 1 g SILICON (UNII: Z4152N8IUI) 0.1 g in 1 g Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:82736-011-01 1 g in 1 CARTON; Type 0: Not a Combination Product 05/26/2022 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 05/26/2022 MATTIFYING FACIAL SPF 15 OILY SKIN
octinoxate creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:82736-013 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 0.1 g in 1 g Inactive Ingredients Ingredient Name Strength OXYBENZONE (UNII: 95OOS7VE0Y) 0.1 g in 1 g AVOBENZONE (UNII: G63QQF2NOX) 0.8 g in 1 g Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:82736-013-01 1 g in 1 CARTON; Type 0: Not a Combination Product 05/26/2022 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 05/26/2022 BODY SUNSCREEN FPS 50
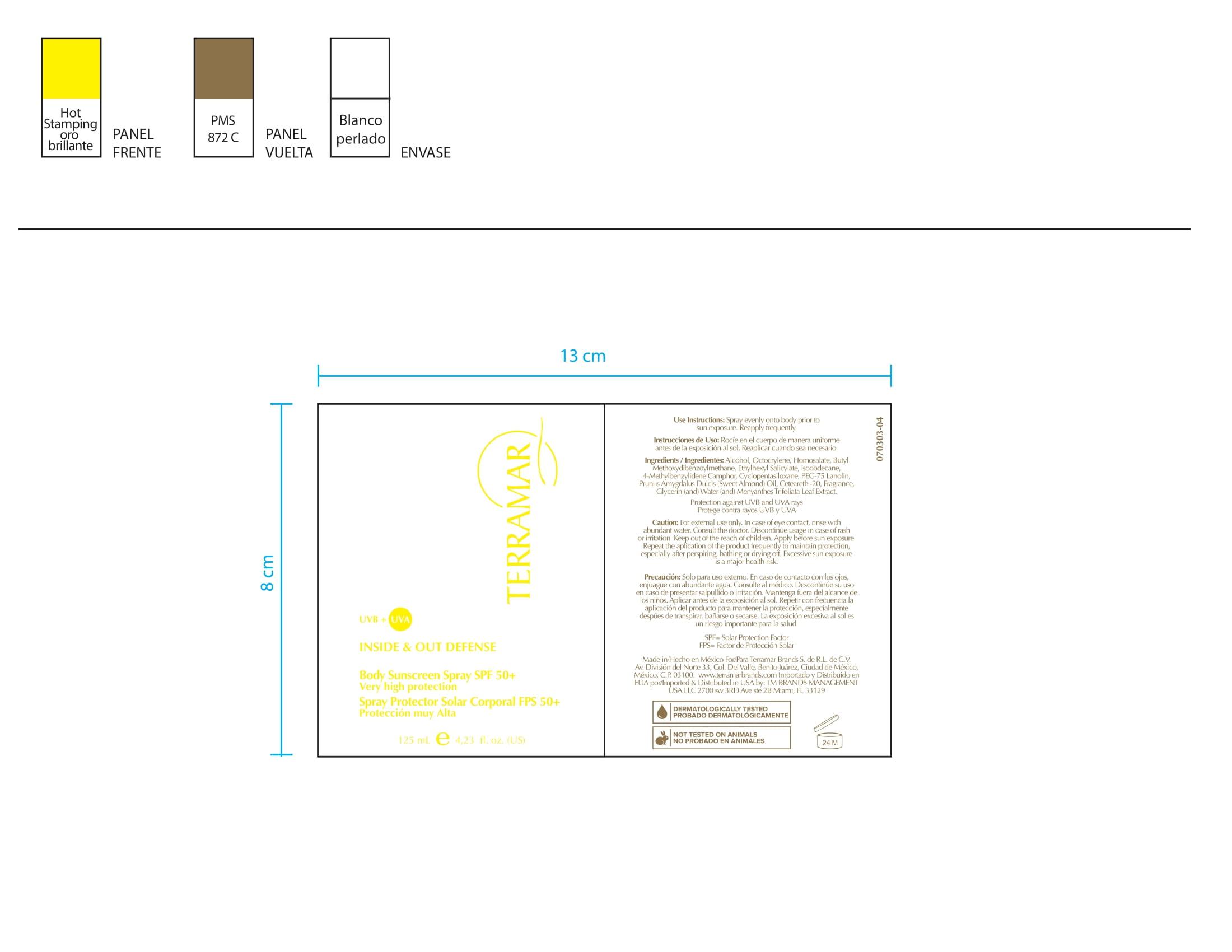
octocrylene, homosalate, butyl methoxydibenzoylmethane, ethylhexyl salicylate, 4-methylbenzylidene camphor creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:82736-014 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 0.1 g in 1 g AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 0.6 g in 1 g Inactive Ingredients Ingredient Name Strength HOMOSALATE (UNII: V06SV4M95S) 0.1 g in 1 g ENZACAMENE (UNII: 8I3XWY40L9) 0.1 g in 1 g OCTINOXATE (UNII: 4Y5P7MUD51) 0.1 g in 1 g Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:82736-014-01 1 g in 1 CARTON; Type 0: Not a Combination Product 05/26/2022 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 05/26/2022 PROTECTIVE MAKEUP SPF 15 WITH EDELWESS STEM CELLS
ethylhexyl methoxycinnamate, benzophenone-3 creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:82736-004 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 0.1 g in 1 g Inactive Ingredients Ingredient Name Strength OXYBENZONE (UNII: 95OOS7VE0Y) 0.4 g in 1 g Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:82736-004-01 1 g in 1 CARTON; Type 0: Not a Combination Product 05/26/2022 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 05/26/2022 MAQUILLAJE LIQUIDO MAXIMA COBERTURA
ethylhexyl methoxycinnamate, benzophenone- 3 creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:82736-006 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 0.1 g in 1 g Inactive Ingredients Ingredient Name Strength OXYBENZONE (UNII: 95OOS7VE0Y) 0.9 g in 1 g Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:82736-006-01 1 g in 1 CARTON; Type 0: Not a Combination Product 05/26/2022 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part352 05/26/2022 MATURE SKIN FACIAL DAY SPF 15
ethylhexyl methoxycinnamate, titanium dioxide, silica, dimethicone creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:82736-005 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 0.1 g in 1 g TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 0.1 g in 1 g Inactive Ingredients Ingredient Name Strength DIMETHICONE (UNII: 92RU3N3Y1O) 0.7 g in 1 g SILICON (UNII: Z4152N8IUI) 0.1 g in 1 g Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:82736-005-01 1 g in 1 CARTON; Type 0: Not a Combination Product 05/26/2022 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 05/26/2022 COLOR CORRECTING SPF 20
ethylhexyl methoxycinnamate, octocrylene, butyl methoxydibenzoylmethane creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:82736-002 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 0.1 g in 1 g Inactive Ingredients Ingredient Name Strength OCTOCRYLENE (UNII: 5A68WGF6WM) 0.8 g in 1 g AVOBENZONE (UNII: G63QQF2NOX) 0.1 g in 1 g Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:82736-002-02 1 g in 1 CARTON; Type 0: Not a Combination Product 05/26/2022 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 05/26/2022 SUN PROTECTION FACIAL SPF 50
homosalate, neo heliopan, ethylhexyl salicylate, benzophenone-3, octocrylene, avovenzona (butyl methoxydibenzoylmethane), titanium dioxide ci 77891 creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:82736-009 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 0.1 g in 1 g Inactive Ingredients Ingredient Name Strength OCTOCRYLENE (UNII: 5A68WGF6WM) 0.1 g in 1 g OXYBENZONE (UNII: 95OOS7VE0Y) 0.5 g in 1 g TITANIUM DIOXIDE (UNII: 15FIX9V2JP) 0.1 g in 1 g OCTISALATE (UNII: 4X49Y0596W) 0.1 g in 1 g AVOBENZONE (UNII: G63QQF2NOX) 0.1 g in 1 g Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:82736-009-01 1 g in 1 CARTON; Type 0: Not a Combination Product 05/26/2022 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 05/26/2022 ANTI HAIR LOSS GEL WITH ARNICA, ROSEMARY, AND LEMON EXTRACTS.
propylene glycol gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:82736-016 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SALICYLIC ACID (UNII: O414PZ4LPZ) (SALICYLIC ACID - UNII:O414PZ4LPZ) SALICYLIC ACID 0.1 g in 1 g Inactive Ingredients Ingredient Name Strength ROSMARINUS OFFICINALIS FLOWERING TOP (UNII: 8JM482TI79) 0.1 g in 1 g CITRUS X LIMON FLOWERING TOP OIL (UNII: 4C38SS0J60) 0.7 g in 1 g PROPYLENE GLYCOL (UNII: 6DC9Q167V3) 0.1 g in 1 g Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:82736-016-01 1 g in 1 CARTON; Type 0: Not a Combination Product 05/26/2022 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final M032 05/26/2022 DESODORANTE ANTIPERSPIRANT
aluminium chlorohydrate aerosol, sprayProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:82736-003 Route of Administration INTRADERMAL, TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALUMINUM CHLOROHYDRATE (UNII: HPN8MZW13M) (ALUMINUM CHLOROHYDRATE - UNII:HPN8MZW13M) ALUMINUM CHLOROHYDRATE 0.24 g in 1 g Inactive Ingredients Ingredient Name Strength BISMUTH SUBCITRATE POTASSIUM (UNII: R3O80H60KX) 0.76 g in 1 g Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:82736-003-02 1 g in 1 CARTON; Type 0: Not a Combination Product 05/26/2022 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final M019 05/26/2022 FACIAL PRIMER PROTECTOR SPF 50
homosalate, benzophenone-3, octocrylene, ethylexyl salicylate, butyl methoxydibenzoylmethane creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:82736-001 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 0.1 g in 1 g OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 0.1 g in 1 g OXYBENZONE (UNII: 95OOS7VE0Y) (OXYBENZONE - UNII:95OOS7VE0Y) OXYBENZONE 0.1 g in 1 g HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 0.1 g in 1 g Inactive Ingredients Ingredient Name Strength AVOBENZONE (UNII: G63QQF2NOX) 0.1 g in 1 g ALUMINUM STARCH OCTENYLSUCCINATE (UNII: I9PJ0O6294) 0.3 g in 1 g HYDROGENATED SOYBEAN LECITHIN (UNII: H1109Z9J4N) 0.1 g in 1 g DIMETHICONE CROSSPOLYMER (450000 MPA.S AT 12% IN CYCLOPENTASILOXANE) (UNII: UF7620L1W6) 0.1 g in 1 g Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:82736-001-01 1 g in 1 CARTON; Type 0: Not a Combination Product 06/01/2022 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 06/01/2022 FOCUSED CORRECTIVE
water (aqua), butylene glycol, peg-60 almond glycerides, caprylyl glycol, glycerin, carbomer, nordihydroguaiaretic acid, oleanolic acid, beta vulgaris (beet) root extract, oenothera biennis (evening primrose) leaf extract creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:82736-007 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 0.2 g in 1 g BUTYLENE GLYCOL (UNII: 3XUS85K0RA) (BUTYLENE GLYCOL - UNII:3XUS85K0RA) BUTYLENE GLYCOL 0.1 g in 1 g Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) 0.1 g in 1 g CAPRYLYL GLYCOL (UNII: 00YIU5438U) 0.1 g in 1 g OENOTHERA BIENNIS (UNII: 76UI55V071) 0.3 g in 1 g PEG-60 ALMOND GLYCERIDES (UNII: 4Y0E651N0F) 0.1 g in 1 g GLYCERIN (UNII: PDC6A3C0OX) 0.1 g in 1 g Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:82736-007-01 1 g in 1 CARTON; Type 0: Not a Combination Product 05/26/2022 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 05/26/2022 Labeler - TERRAMAR BRANDS S de RL de CV (588108527) Registrant - TERRAMAR BRANDS S de RL de CV (588108527) Establishment Name Address ID/FEI Business Operations TERRAMAR BRAND S de RL de CV 588108527 manufacture(82736-008, 82736-010, 82736-019, 82736-020, 82736-012, 82736-015, 82736-018, 82736-017, 82736-011, 82736-013, 82736-014, 82736-004, 82736-006, 82736-005, 82736-002, 82736-009, 82736-016, 82736-003, 82736-001, 82736-007)