Label: ANTI ACNE EXFOLIATING CLEANSING PADS- salicylic acid swab
-
Contains inactivated NDC Code(s)
NDC Code(s): 65903-242-01 - Packager: H2O Plus
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated September 28, 2011
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
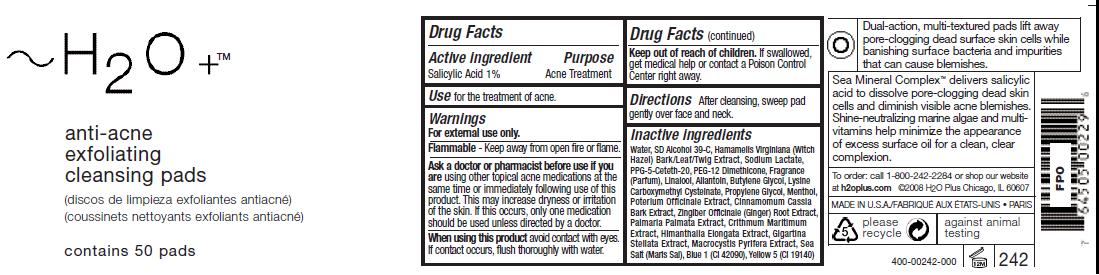
ACTIVE INGREDIENT
Active ingredient
Salicylic Acid 1%Ask a doctor or pharmacist before use if you
are using other topical acne medications at the
same time or immediately following use of this
product. This may increase dryness or irritation
of the skin. If this occurs, only one medication
should be used unless directed by a doctor.Keep out of reach of children. If swallowed,
get medical help or contact a Poison Control
Center right away.
Inactive ingredients
Water, SD Alcohol 39-C, Hamamelis Virginiana (Witch
Hazel) Bark/Leaf/Twig Extract, Sodium Lactate,
PPG-5-Ceteth-20, PEG-12 Dimethicone, Fragrance
(Parfum), Linalool, Allantoin, Butylene Glycol, Lysine
Carboxymethyl Cysteinate, Propylene Glycol, Menthol,
Poterium Officinale Extract, Cinnamomum Cassia
Bark Extract, Zingiber Officinale (Ginger) Root Extract,
Palmaria Palmata Extract, Crithmum Maritimum
Extract, Himanthalia Elongata Extract, Gigartina
Stellata Extract, Macrocystis Pyrifera Extract, Sea
Salt (Maris Sal), Blue 1 (CI 42090), Yellow 5 (CI 19140) - PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
ANTI ACNE EXFOLIATING CLEANSING PADS
salicylic acid swabProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:65903-242 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Salicylic Acid (UNII: O414PZ4LPZ) (Salicylic Acid - UNII:O414PZ4LPZ) Salicylic Acid 10 uL in 1 mL Inactive Ingredients Ingredient Name Strength Water (UNII: 059QF0KO0R) ALCOHOL (UNII: 3K9958V90M) HAMAMELIS VIRGINIANA BARK (UNII: IH3063S9MY) SODIUM LACTATE (UNII: TU7HW0W0QT) PPG-5-CETETH-20 (UNII: 4AAN25P8P4) PEG-12 DIMETHICONE (UNII: ZEL54N6W95) LINALOOL, (+/-)- (UNII: D81QY6I88E) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) CARBOCYSTEINE LYSINE (UNII: 1D1Y95PXXA) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) MENTHOL (UNII: L7T10EIP3A) SANGUISORBA MINOR ROOT (UNII: 11YGU8I0TT) CHINESE CINNAMON (UNII: WS4CQ062KM) GINGER (UNII: C5529G5JPQ) DULSE (UNII: 7832HOY4ZQ) CRITHMUM MARITIMUM (UNII: J7IHY79BKY) HIMANTHALIA ELONGATA (UNII: 21RND18XRR) MASTOCARPUS STELLATUS (UNII: 6T087FC66H) MACROCYSTIS PYRIFERA (UNII: K31S3OG5C4) SEA SALT (UNII: 87GE52P74G) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:65903-242-01 57 mL in 1 JAR Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part333D 09/01/2008 Labeler - H2O Plus (807722947) Registrant - H2O Plus (807722947) Establishment Name Address ID/FEI Business Operations H2O Plus 807722947 manufacture