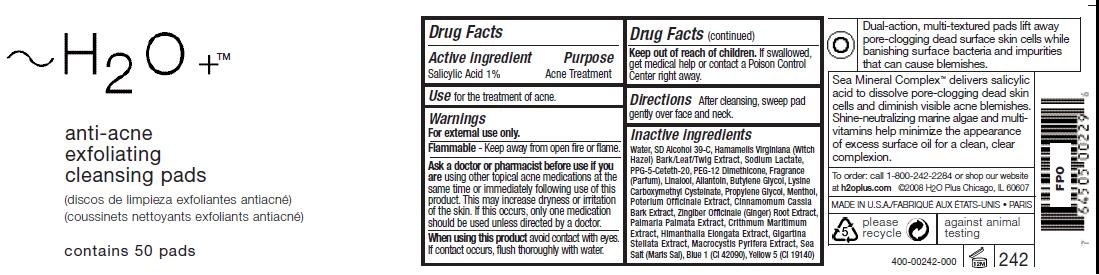
Active ingredient
Salicylic Acid 1%
Ask a doctor or pharmacist before use if you
are using other topical acne medications at the
same time or immediately following use of this
product. This may increase dryness or irritation
of the skin. If this occurs, only one medication
should be used unless directed by a doctor.
Keep out of reach of children. If swallowed,
get medical help or contact a Poison Control
Center right away.
Inactive ingredients
Water, SD Alcohol 39-C, Hamamelis Virginiana (Witch
Hazel) Bark/Leaf/Twig Extract, Sodium Lactate,
PPG-5-Ceteth-20, PEG-12 Dimethicone, Fragrance
(Parfum), Linalool, Allantoin, Butylene Glycol, Lysine
Carboxymethyl Cysteinate, Propylene Glycol, Menthol,
Poterium Officinale Extract, Cinnamomum Cassia
Bark Extract, Zingiber Officinale (Ginger) Root Extract,
Palmaria Palmata Extract, Crithmum Maritimum
Extract, Himanthalia Elongata Extract, Gigartina
Stellata Extract, Macrocystis Pyrifera Extract, Sea
Salt (Maris Sal), Blue 1 (CI 42090), Yellow 5 (CI 19140)