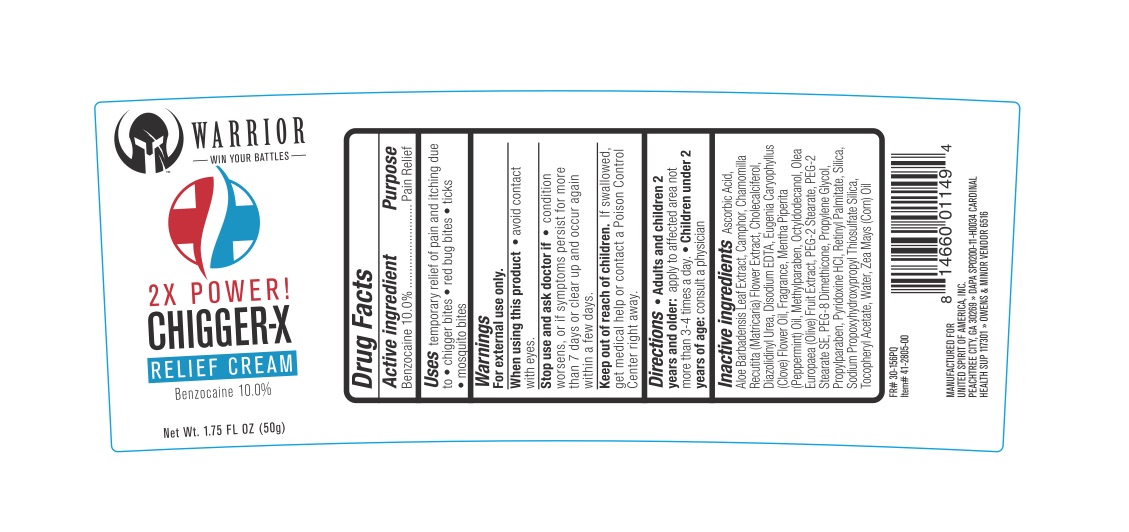
Label: WARRIOR CHIGGEREX RELIEF- benzocaine 10% cream
- NDC Code(s): 72839-069-01
- Packager: Derma Care Research Labs, LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated April 18, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
- DOSAGE & ADMINISTRATION
- WARNINGS
- KEEP OUT OF REACH OF CHILDREN
- INDICATIONS & USAGE
-
INACTIVE INGREDIENT
Ascorbic Acid, Aloe Barbadensis Leaf Extract, Camphor, Chamomilla Recutita (Matricaria) Flower Extract, Cholecalciferol, Diazolidinyl Urea, Disodium EDTA, Eugenia Caryophyllus (Clove) Flower Oil, Fragrance, Mentha Piperita (Peppermint) Oil, Methylparaben, Octyldodecanol, Olea Europaea (Olive) Fruit Extract, PEG-2 Stearate, PEG-2 Stearate SE, PEG-8 Dimethicone, Propylene Glycol, Propylparaben, Pyridoxine HCl, Retinyl Palmitate, Silica, Sodium Propoxyhydroxypropyl Thiosulfate Silica, Tocopheryl Acetate, Water, Zea Mays (Corn) Oil.
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
WARRIOR CHIGGEREX RELIEF
benzocaine 10% creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:72839-069 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZOCAINE (UNII: U3RSY48JW5) (BENZOCAINE - UNII:U3RSY48JW5) BENZOCAINE 10 g in 100 g Inactive Ingredients Ingredient Name Strength CAMPHOR (SYNTHETIC) (UNII: 5TJD82A1ET) PEG-2 STEARATE (UNII: 94YQ11Y95F) CLOVE OIL (UNII: 578389D6D0) MATRICARIA CHAMOMILLA FLOWERING TOP OIL (UNII: SA8AR2W4ER) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) OCTYLDODECANOL (UNII: 461N1O614Y) SODIUM PROPOXYHYDROXYPROPYL THIOSULFATE SILICA (UNII: 208G222332) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) PEPPERMINT OIL (UNII: AV092KU4JH) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) VITAMIN A PALMITATE (UNII: 1D1K0N0VVC) ALOE VERA LEAF (UNII: ZY81Z83H0X) DIAZOLIDINYL UREA (UNII: H5RIZ3MPW4) 4-DESOXYPYRIDOXINE HYDROCHLORIDE (UNII: P9QAN95HHX) EDETATE DISODIUM (UNII: 7FLD91C86K) CORN OIL (UNII: 8470G57WFM) ASCORBIC ACID (UNII: PQ6CK8PD0R) OLIVE OIL (UNII: 6UYK2W1W1E) METHYLPARABEN (UNII: A2I8C7HI9T) PEG-8 DIMETHICONE (UNII: GIA7T764OD) WATER (UNII: 059QF0KO0R) CHOLECALCIFEROL (UNII: 1C6V77QF41) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:72839-069-01 50 g in 1 JAR; Type 0: Not a Combination Product 08/14/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part348 08/14/2020 Labeler - Derma Care Research Labs, LLC (116817470) Registrant - Derma Care Research Labs, LLC (116817470) Establishment Name Address ID/FEI Business Operations Derma Care Research Labs, LLC 116817470 manufacture(72839-069)