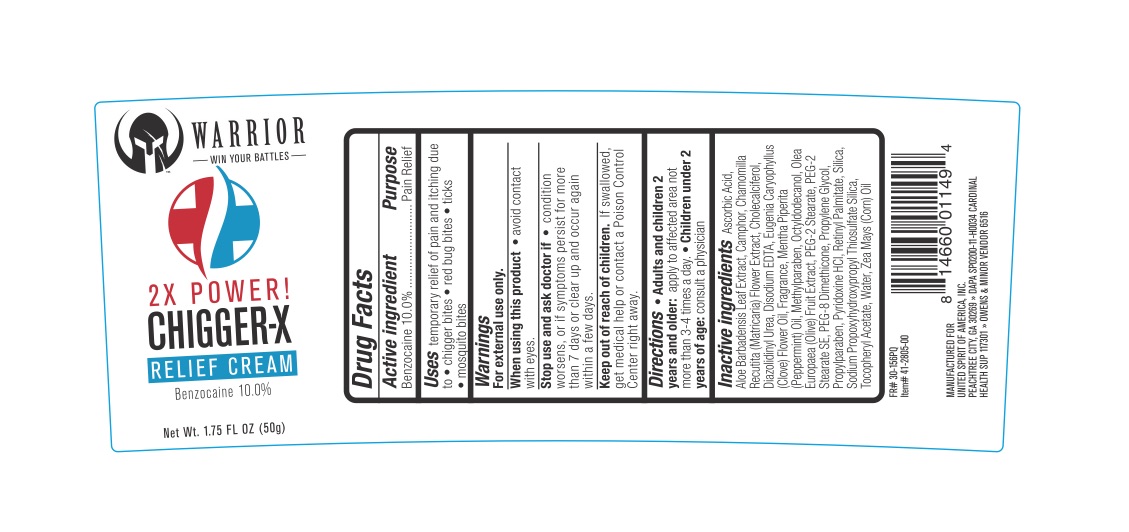
Uses: for the temporary relief of pain and itching associated with chigger bites, red bug bites, ticks, and mosquito bites.
For external use only.
When using this product avoid contact with eyes. Stop use and ask a doctor if the condition worsens, symptoms persist for more than 7 days or clear up and occur again within a few days.
In case of accidental ingestion, seek professional assistance or contact a Poison Control Center immediately.
Adults and children 2 years and older: apply to the affected area, not more than 3 to 4 times daily. Children under 2 years of age: ask a doctor.
Ascorbic Acid, Aloe Barbadensis Leaf Extract, Camphor, Chamomilla Recutita (Matricaria) Flower Extract, Cholecalciferol, Diazolidinyl Urea, Disodium EDTA, Eugenia Caryophyllus (Clove) Flower Oil, Fragrance, Mentha Piperita (Peppermint) Oil, Methylparaben, Octyldodecanol, Olea Europaea (Olive) Fruit Extract, PEG-2 Stearate, PEG-2 Stearate SE, PEG-8 Dimethicone, Propylene Glycol, Propylparaben, Pyridoxine HCl, Retinyl Palmitate, Silica, Sodium Propoxyhydroxypropyl Thiosulfate Silica, Tocopheryl Acetate, Water, Zea Mays (Corn) Oil.