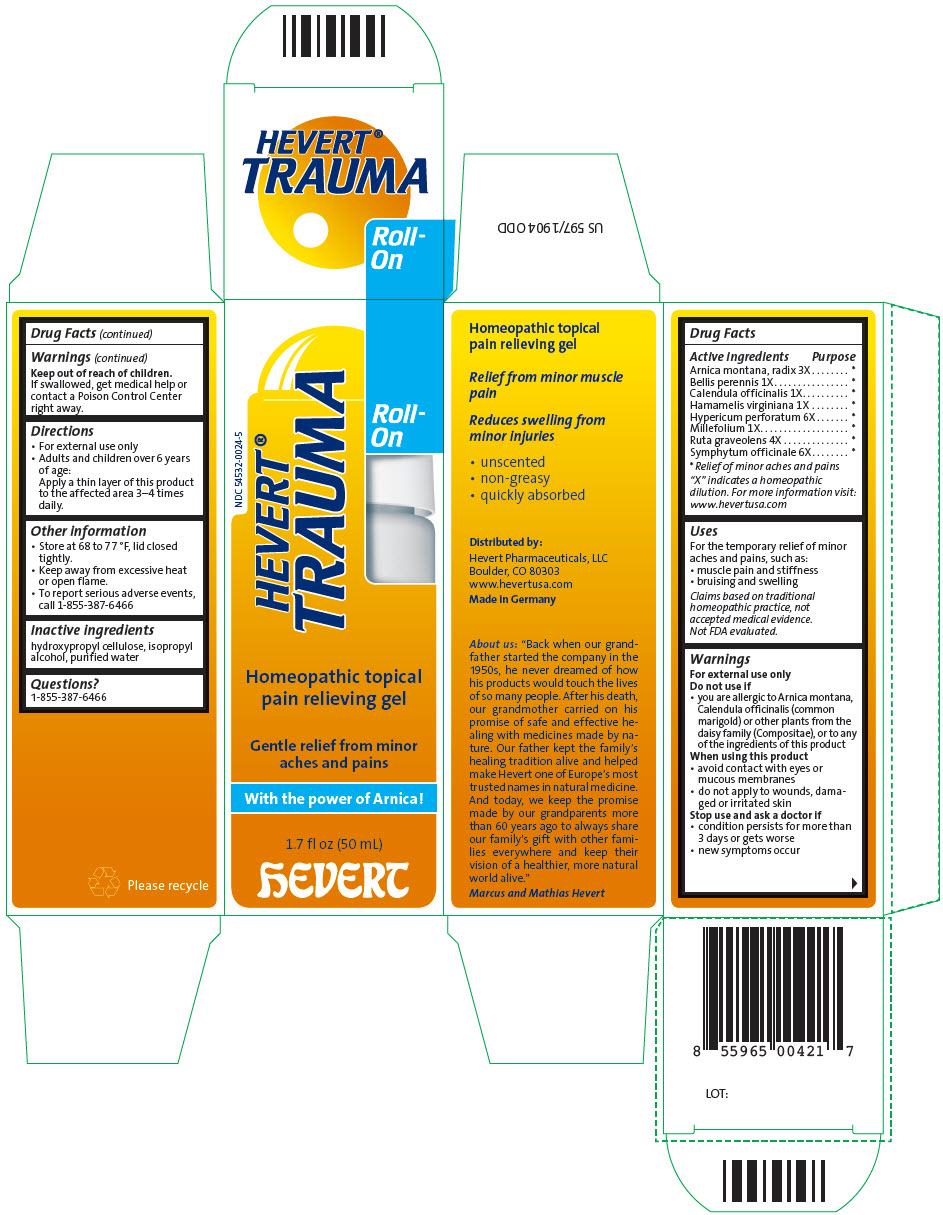
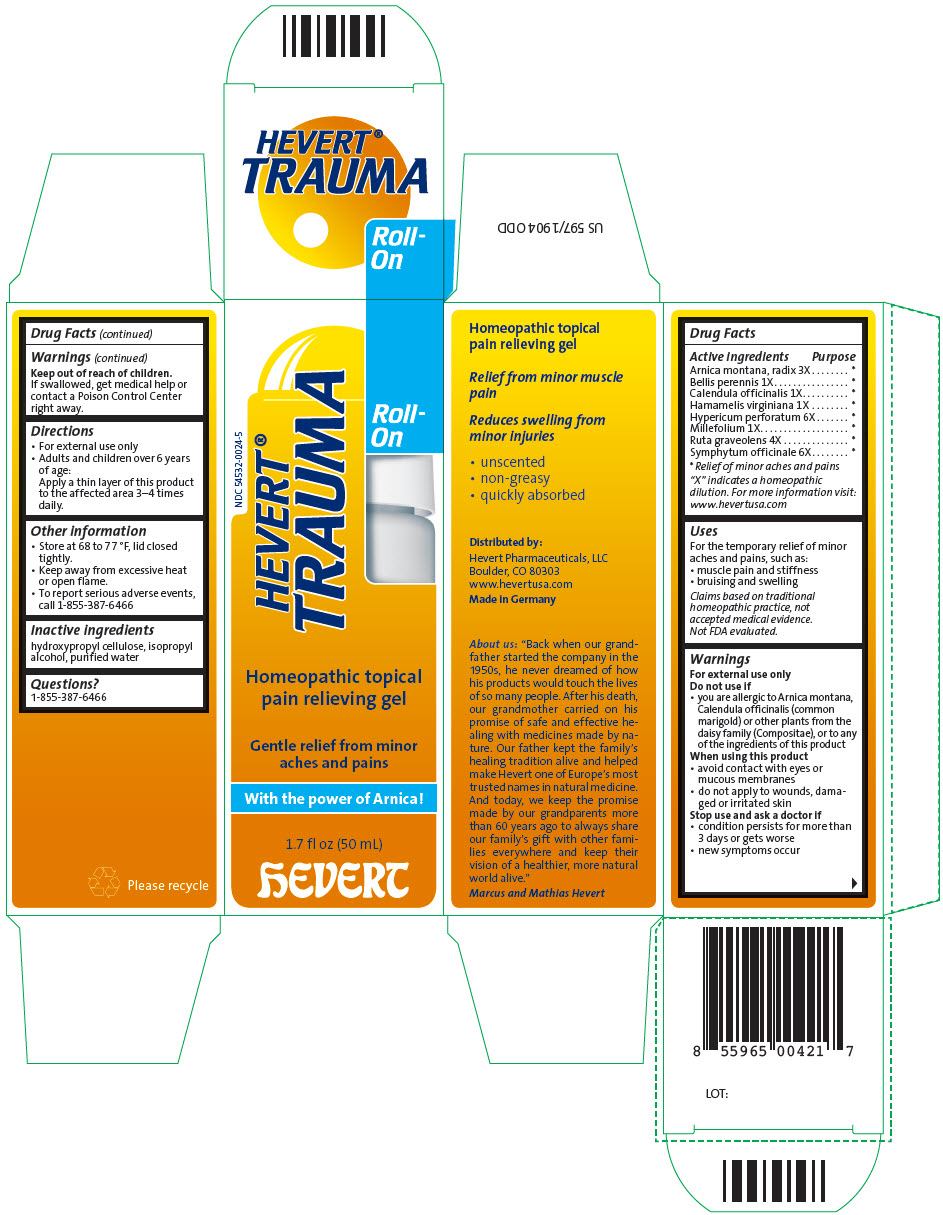
Label: HEVERT TRAUMA ROLL-ON- arnica montana root, bellis perennis, calendula officinalis flowering top, hamamelis virginiana root bark/stem bark, hypericum perforatum, achillea millefolium flowering top, ruta graveolens flowering top, and comfrey root gel
- NDC Code(s): 54532-0024-5, 54532-0024-6
- Packager: Hevert Arzneimittel GmbH & Co KG
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved homeopathic
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated December 15, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
-
ACTIVE INGREDIENT
Active ingredients Purpose - *
- Relief of minor aches and pains
Arnica montana, radix 3X * Bellis perennis 1X * Calendula officinalis 1X * Hamamelis virginiana 1X * Hypericum perforatum 6X * Millefolium 1X * Ruta graveolens 4X * Symphytum officinale 6X * "X" indicates a homeopathic dilution. For more information visit: www.hevertusa.com
- Uses
-
Warnings
For external use only
Do not use if
- you are allergic to Arnica montana, Calendula officinalis (common marigold) or other plants from the daisy family (Compositae), or to any of the ingredients of this product
When using this product
- avoid contact with eyes or mucous membranes
- do not apply to wounds, damaged or irritated skin
- Directions
- Other information
- Inactive ingredients
- Questions?
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 50 mL Bottle Carton
-
INGREDIENTS AND APPEARANCE
HEVERT TRAUMA ROLL-ON
arnica montana root, bellis perennis, calendula officinalis flowering top, hamamelis virginiana root bark/stem bark, hypericum perforatum, achillea millefolium flowering top, ruta graveolens flowering top, and comfrey root gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:54532-0024 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ARNICA MONTANA ROOT (UNII: MUE8Y11327) (ARNICA MONTANA ROOT - UNII:MUE8Y11327) ARNICA MONTANA ROOT 3 [hp_X] in 1 mL BELLIS PERENNIS WHOLE (UNII: 2HU33I03UY) (BELLIS PERENNIS WHOLE - UNII:2HU33I03UY) BELLIS PERENNIS WHOLE 1 [hp_X] in 1 mL CALENDULA OFFICINALIS FLOWERING TOP (UNII: 18E7415PXQ) (CALENDULA OFFICINALIS FLOWERING TOP - UNII:18E7415PXQ) CALENDULA OFFICINALIS FLOWERING TOP 1 [hp_X] in 1 mL HAMAMELIS VIRGINIANA ROOT BARK/STEM BARK (UNII: T7S323PKJS) (HAMAMELIS VIRGINIANA ROOT BARK/STEM BARK - UNII:T7S323PKJS) HAMAMELIS VIRGINIANA ROOT BARK/STEM BARK 1 [hp_X] in 1 mL HYPERICUM PERFORATUM WHOLE (UNII: XK4IUX8MNB) (HYPERICUM PERFORATUM WHOLE - UNII:XK4IUX8MNB) HYPERICUM PERFORATUM WHOLE 6 [hp_X] in 1 mL ACHILLEA MILLEFOLIUM FLOWERING TOP (UNII: 55862Q3XEU) (ACHILLEA MILLEFOLIUM FLOWERING TOP - UNII:55862Q3XEU) ACHILLEA MILLEFOLIUM FLOWERING TOP 1 [hp_X] in 1 mL RUTA GRAVEOLENS FLOWERING TOP (UNII: N94C2U587S) (RUTA GRAVEOLENS FLOWERING TOP - UNII:N94C2U587S) RUTA GRAVEOLENS FLOWERING TOP 4 [hp_X] in 1 mL COMFREY ROOT (UNII: M9VVZ08EKQ) (COMFREY ROOT - UNII:M9VVZ08EKQ) COMFREY ROOT 6 [hp_X] in 1 mL Inactive Ingredients Ingredient Name Strength HYDROXYPROPYL CELLULOSE (1600000 WAMW) (UNII: RFW2ET671P) ISOPROPYL ALCOHOL (UNII: ND2M416302) WATER (UNII: 059QF0KO0R) Product Characteristics Color YELLOW (weak yellow) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:54532-0024-5 1 in 1 CARTON 04/01/2016 1 50 mL in 1 BOTTLE, WITH APPLICATOR; Type 0: Not a Combination Product 2 NDC:54532-0024-6 1 in 1 CARTON 04/01/2016 2 10 mL in 1 BOTTLE, WITH APPLICATOR; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date UNAPPROVED HOMEOPATHIC 04/01/2016 Labeler - Hevert Arzneimittel GmbH & Co KG (318100617) Establishment Name Address ID/FEI Business Operations Hevert Arzneimittel GmbH & Co. KG 318100617 MANUFACTURE(54532-0024)