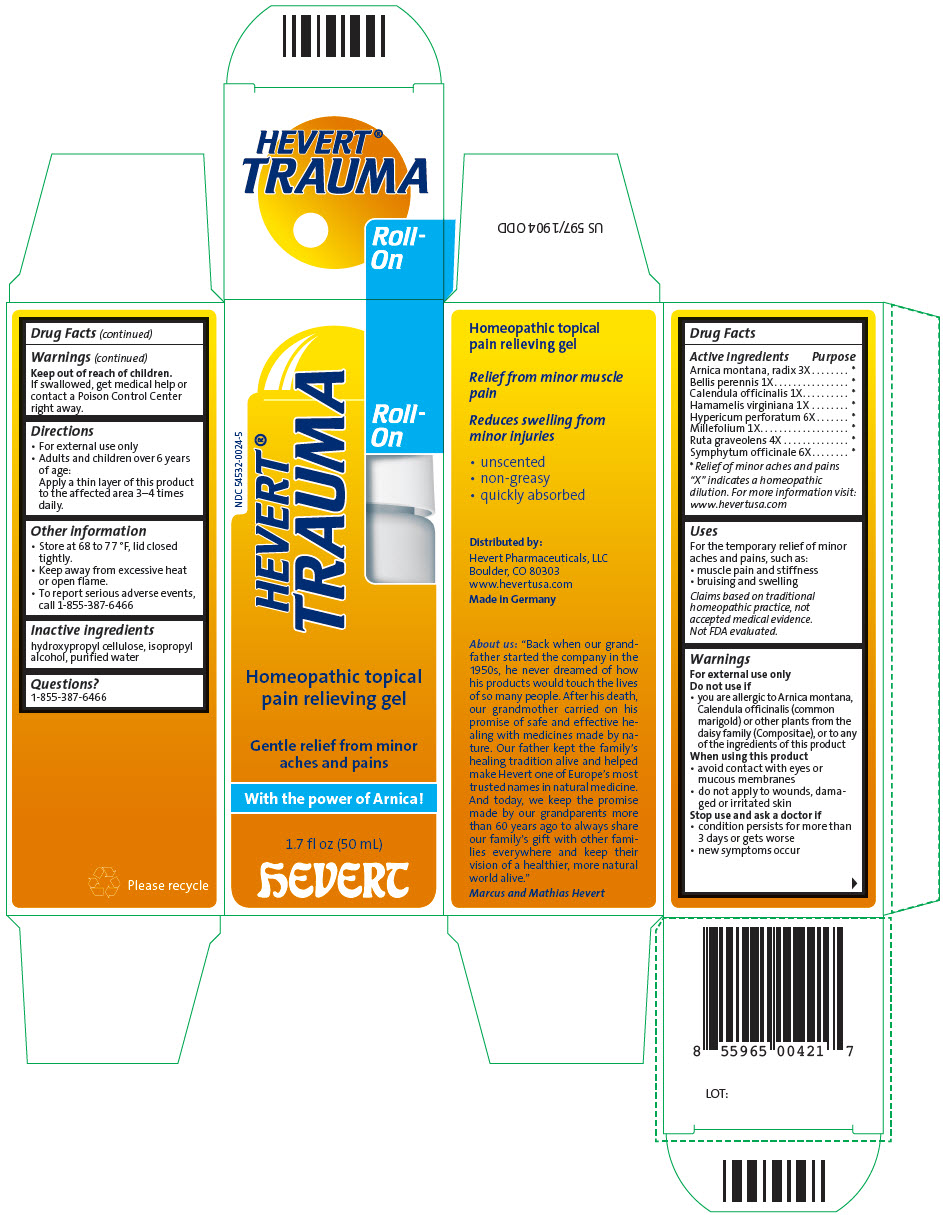
HEVERT TRAUMA ROLL-ON- arnica montana root, bellis perennis, calendula officinalis flowering top, hamamelis virginiana root bark/stem bark, hypericum perforatum, achillea millefolium flowering top, ruta graveolens flowering top, and comfrey root gel
Hevert Arzneimittel GmbH & Co KG
Disclaimer: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
----------
| Active ingredients | Purpose |
|
|
| Arnica montana, radix 3X | * |
| Bellis perennis 1X | * |
| Calendula officinalis 1X | * |
| Hamamelis virginiana 1X | * |
| Hypericum perforatum 6X | * |
| Millefolium 1X | * |
| Ruta graveolens 4X | * |
| Symphytum officinale 6X | * |
"X" indicates a homeopathic dilution. For more information visit: www.hevertusa.com
Uses
For the temporary relief of minor aches and pains, such as:
- muscle pain and stiffness
- bruising and swelling
Claims based on traditional homeopathic practice, not accepted medical evidence. Not FDA evaluated.
Warnings
For external use only
Do not use if
- you are allergic to Arnica montana, Calendula officinalis (common marigold) or other plants from the daisy family (Compositae), or to any of the ingredients of this product
When using this product
- avoid contact with eyes or mucous membranes
- do not apply to wounds, damaged or irritated skin
Stop use and ask a doctor if
- condition persists for more than 3 days or gets worse
- new symptoms occur
Keep out of reach of children.
If swallowed, get medical help or contact a Poison Control Center right away.
Directions
- For external use only
- Adults and children over 6 years of age:
Apply a thin layer of this product to the affected area 3–4 times daily.
Other information
- Store at 68 to 77 °F, lid closed tightly.
- Keep away from excessive heat or open flame.
- To report serious adverse events, call 1-855-387-6466
Inactive ingredients
hydroxypropyl cellulose, isopropyl alcohol, purified water
Questions?
1-855-387-6466
Distributed by:
Hevert Pharmaceuticals, LLC
Boulder, CO 80303
PRINCIPAL DISPLAY PANEL - 50 mL Bottle Carton
NDC 54532-0024-5
HEVERT®
TRAUMA
Roll-
On
Homeopathic topical
pain relieving gel
Gentle relief from minor
aches and pains
With the power of Arnica !
1.7 fl oz (50 mL)
hEVERT