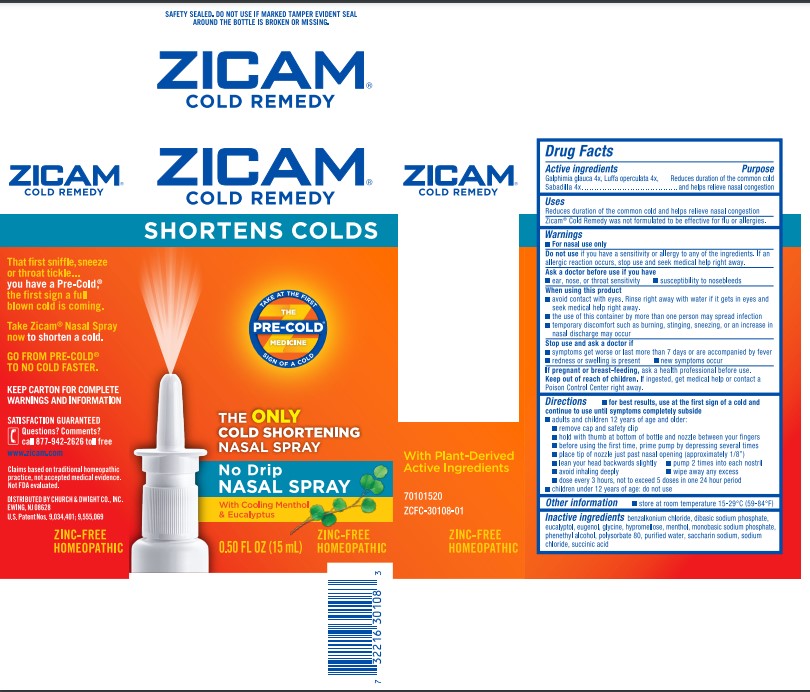
Label: ZICAM COLD REMEDY- galphimia glauca flowering top, luffa operculata fruit, and schoenocaulon officinale seed spray
- NDC Code(s): 10237-474-15
- Packager: Church & Dwight Co., Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved homeopathic
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated April 10, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active ingredients
- Purpose
- Uses
-
Warnings
- For nasal use only
Do not use if you have a sensitivity or allergy to any of the ingredients. If an allergic reaction occurs, stop use and seek medical help right away.
When using this product
- avoid contact with eyes. Rinse right away with water if it gets in eyes and seek medical help right away.
- the use of this container by more than one person may spread infection
- temporary discomfort such as burning, stinging, sneezing, or an increase in nasal discharge may occur
-
Directions
- for best results, use at the first sign of a cold and continue to use until symptoms completely subside
- adults and children 12 years of age and older:
- remove cap and safety clip
- hold with thumb at bottom of bottle and nozzle between your fingers
- before using the first time, prime pump by depressing several times
- place tip of nozzle just past nasal opening (approximately 1/8")
- lean your head backwards slightly
- pump 2 times into each nostril
- avoid inhaling deeply
- wipe away any excess
- dose every 3 hours, not to exceed 5 doses in one 24 hour period
- children under 12 years of age: do not use
- Other information
- Inactive ingredients
- Questions? Comments?
- PRINCIPAL DISPLAY PANEL - 15 mL Bottle Carton
-
INGREDIENTS AND APPEARANCE
ZICAM COLD REMEDY
galphimia glauca flowering top, luffa operculata fruit, and schoenocaulon officinale seed sprayProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:10237-474 Route of Administration NASAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength GALPHIMIA GLAUCA FLOWERING TOP (UNII: 93PH5Q8M7E) (GALPHIMIA GLAUCA FLOWERING TOP - UNII:93PH5Q8M7E) GALPHIMIA GLAUCA FLOWERING TOP 4 [hp_X] in 0.56 mL LUFFA OPERCULATA FRUIT (UNII: C4MO6809HU) (LUFFA OPERCULATA FRUIT - UNII:C4MO6809HU) LUFFA OPERCULATA FRUIT 4 [hp_X] in 0.56 mL SCHOENOCAULON OFFICINALE SEED (UNII: 6NAF1689IO) (SCHOENOCAULON OFFICINALE SEED - UNII:6NAF1689IO) SCHOENOCAULON OFFICINALE SEED 4 [hp_X] in 0.56 mL Inactive Ingredients Ingredient Name Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) SODIUM PHOSPHATE, DIBASIC, UNSPECIFIED FORM (UNII: GR686LBA74) EUCALYPTOL (UNII: RV6J6604TK) EUGENOL (UNII: 3T8H1794QW) GLYCINE (UNII: TE7660XO1C) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) MENTHOL, UNSPECIFIED FORM (UNII: L7T10EIP3A) SODIUM PHOSPHATE, MONOBASIC, UNSPECIFIED FORM (UNII: 3980JIH2SW) PHENYLETHYL ALCOHOL (UNII: ML9LGA7468) POLYSORBATE 80 (UNII: 6OZP39ZG8H) WATER (UNII: 059QF0KO0R) SACCHARIN SODIUM (UNII: SB8ZUX40TY) SODIUM CHLORIDE (UNII: 451W47IQ8X) SUCCINIC ACID (UNII: AB6MNQ6J6L) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:10237-474-15 1 in 1 CARTON 04/10/2022 1 15 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 04/10/2022 Labeler - Church & Dwight Co., Inc. (001211952)