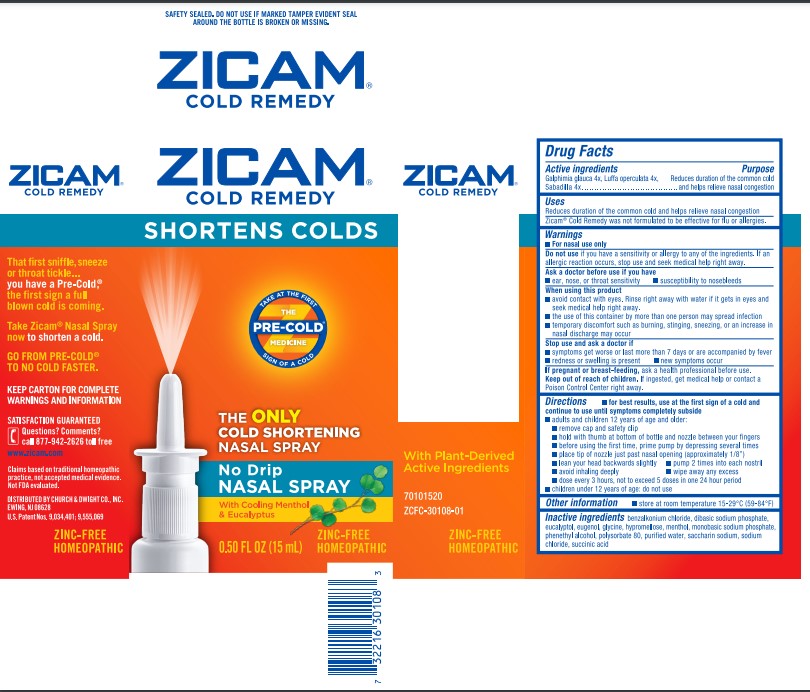
ZICAM COLD REMEDY- galphimia glauca flowering top, luffa operculata fruit, and schoenocaulon officinale seed spray
Church & Dwight Co., Inc.
Disclaimer: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
----------
Active ingredients
Galphimia glauca 4x, Luffa operculata 4x, Sabadilla 4x
Purpose
Reduces duration of the common cold and helps relieve nasal congestion
Uses
Reduces duration of the common cold and helps relieve nasal congestion
---------------------------------------------------------------------------------------------------
Zicam
® Cold Remedy was not formulated to be effective for flu or allergies.
Warnings
Do not use if you have a sensitivity or allergy to any of the ingredients. If an allergic reaction occurs, stop use and seek medical help right away.
Ask a doctor before use if you have
- ear, nose, or throat sensitivity
- susceptibility to nosebleeds
When using this product
- avoid contact with eyes. Rinse right away with water if it gets in eyes and seek medical help right away.
- the use of this container by more than one person may spread infection
- temporary discomfort such as burning, stinging, sneezing, or an increase in nasal discharge may occur
Stop use and ask a doctor if
- symptoms get worse or last more than 7 days or are accompanied by fever
- redness or swelling is present
- new symptoms occur
If pregnant or breast-feeding, ask a health professional before use.
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Directions
-
for best results, use at the first sign of a cold and continue to use until symptoms completely subside
- adults and children 12 years of age and older:
- remove cap and safety clip
- hold with thumb at bottom of bottle and nozzle between your fingers
- before using the first time, prime pump by depressing several times
- place tip of nozzle just past nasal opening (approximately 1/8")
- lean your head backwards slightly
- pump 2 times into each nostril
- avoid inhaling deeply
- wipe away any excess
- dose every 3 hours, not to exceed 5 doses in one 24 hour period
- children under 12 years of age: do not use
Other information
- store at room temperature 15-29°C (59-84°F)
Inactive ingredients
benzalkonium chloride, dibasic sodium phosphate, eucalyptol, eugenol, glycine, hypromellose, menthol, monobasic sodium phosphate, phenethyl alcohol, polysorbate 80, purified water, saccharin sodium, sodium chloride, succinic acid
Questions? Comments?
call 877-942-2626 toll free
www.zicam.com
PRINCIPAL DISPLAY PANEL - 15 mL Bottle Carton
ZICAM
®
COLD REMEDY
SHORTENS COLDS
TAKE AT THE FIRST
THE
PRE-COLD
®
MEDICINE
SIGN OF A COLD
THE
ONLY
COLD SHORTENING
NASAL SPRAY
No Drip
NASAL SPRAY
With Cooling Menthol
& Eucalyptus
0.50 FL OZ (15 mL)
ZINC-FREE
HOMEOPATHIC
Church & Dwight Co., Inc.
