Label: VIGEN DR. NATTOKINASE- natto bacillus, banaba leaf tablet
-
Contains inactivated NDC Code(s)
NDC Code(s): 82437-030-01, 82437-030-02, 82437-030-03 - Packager: Vigen medical
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated December 15, 2021
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
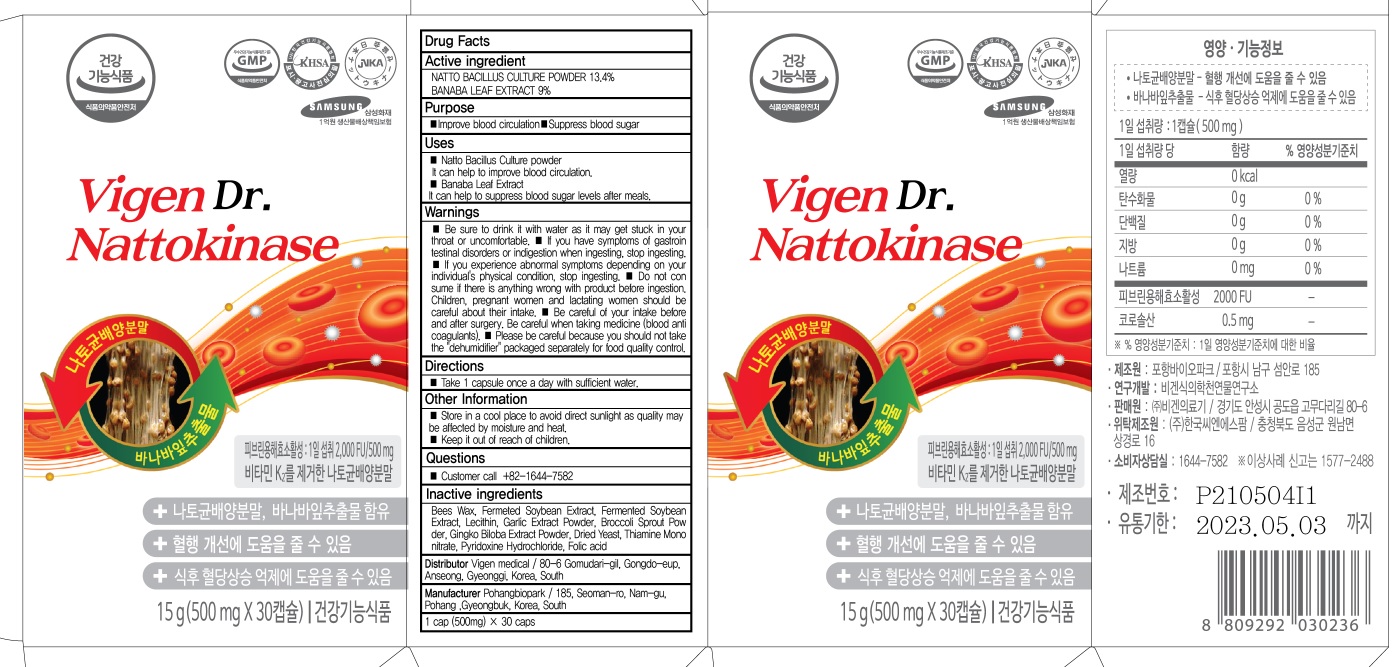
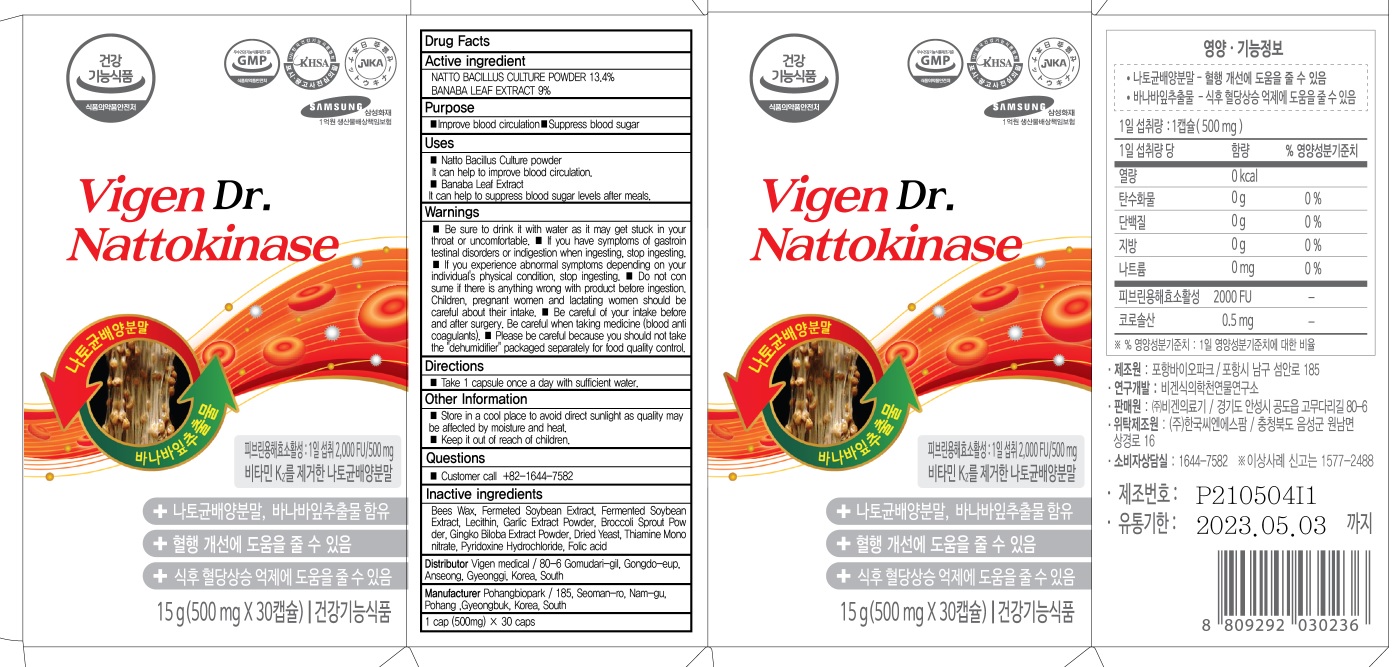
- ACTIVE INGREDIENT
- INACTIVE INGREDIENTS
- PURPOSE
-
WARNINGS
■ Be sure to drink it with water as it may get stuck in your throat or uncomfortable.
■ If you have symptoms of gastrointestinal disorders or indigestion when ingesting, stop ingesting.
■ If you experience abnormal symptoms depending on your individual's physical condition, stop ingesting.
■ Do not consume if there is anything wrong with product before ingestion. Children, pregnant women and lactating women should be careful about their intake.
■ Be careful of your intake before and after surgery. Be careful when taking medicine (blood anticoagulants).
■ Please be careful because you should not take the "dehumidifier" packaged separately for food quality control. - KEEP OUT OF REACH OF CHILDREN
- Uses
- Directions
- Other Information
- Questions
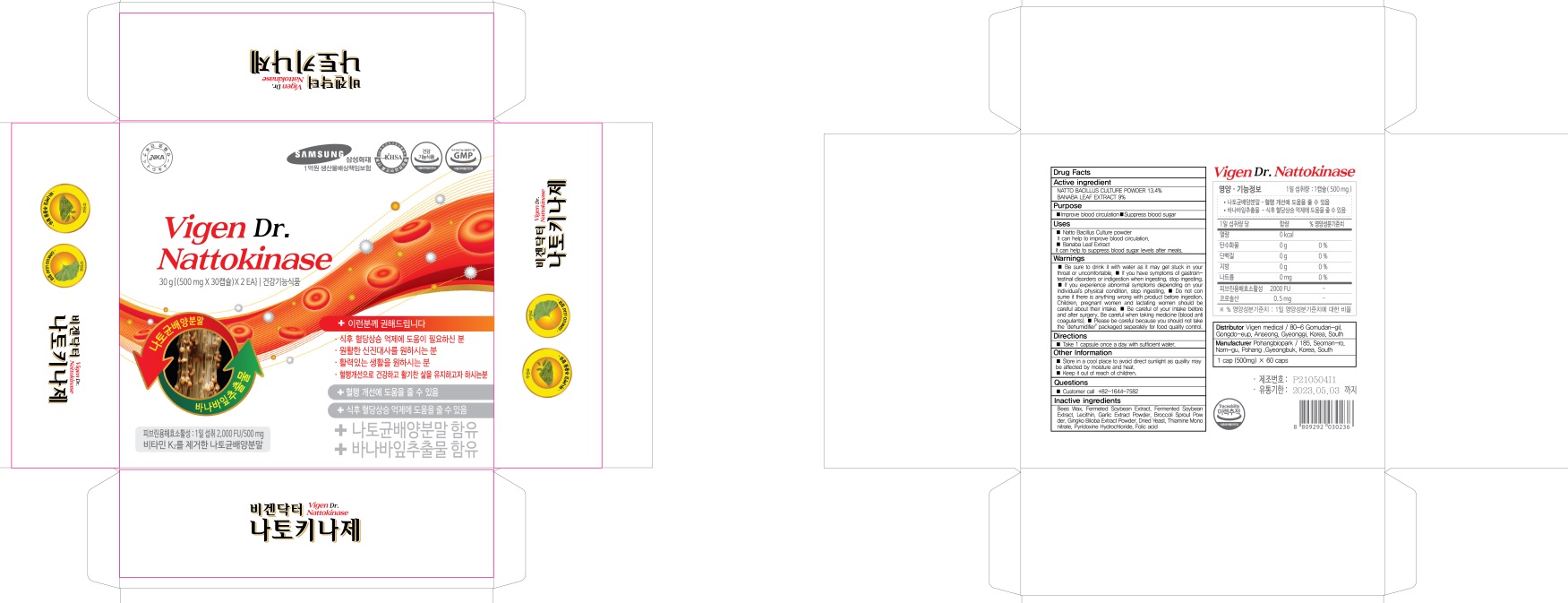
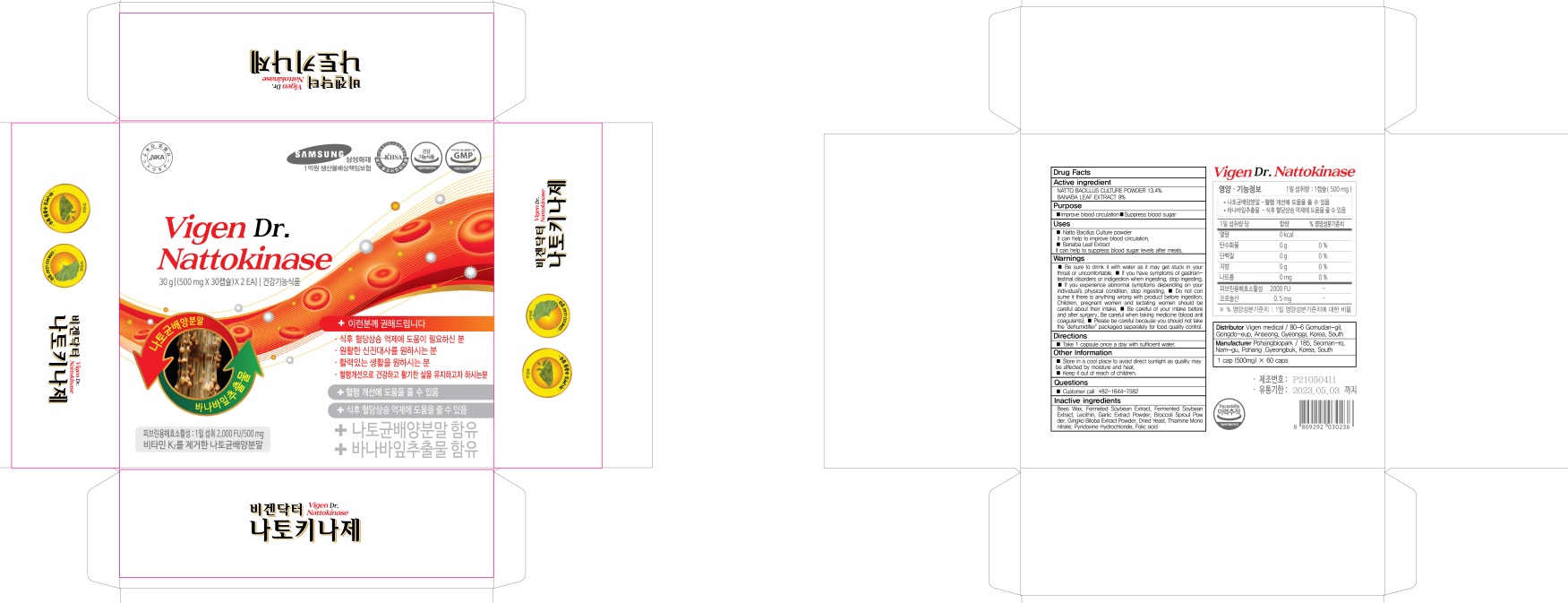
- PACKAGE LABEL82437-030-02 : 30 tablets
- PACKAGE LABEL82437-030-03 : 60 tablets
-
INGREDIENTS AND APPEARANCE
VIGEN DR. NATTOKINASE
natto bacillus, banaba leaf tabletProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:82437-030 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength NATTOKINASE (UNII: H81695M5OP) (NATTOKINASE - UNII:H81695M5OP) NATTOKINASE 67 mg in 500 mg LAGERSTROEMIA SPECIOSA LEAF (UNII: OUK5B3W9E4) (LAGERSTROEMIA SPECIOSA LEAF - UNII:OUK5B3W9E4) LAGERSTROEMIA SPECIOSA LEAF 45 mg in 500 mg Inactive Ingredients Ingredient Name Strength YELLOW WAX (UNII: 2ZA36H0S2V) SOYBEAN OIL (UNII: 241ATL177A) Product Characteristics Color brown Score no score Shape OVAL Size 14mm Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:82437-030-02 30 in 1 CARTON 12/01/2021 1 NDC:82437-030-01 500 mg in 1 BLISTER PACK; Type 0: Not a Combination Product 2 NDC:82437-030-03 60 in 1 CARTON 12/01/2021 2 NDC:82437-030-01 500 mg in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 12/01/2021 Labeler - Vigen medical (688763572) Registrant - Vigen medical (688763572) Establishment Name Address ID/FEI Business Operations Pohangbiopark 688385519 manufacture(82437-030)