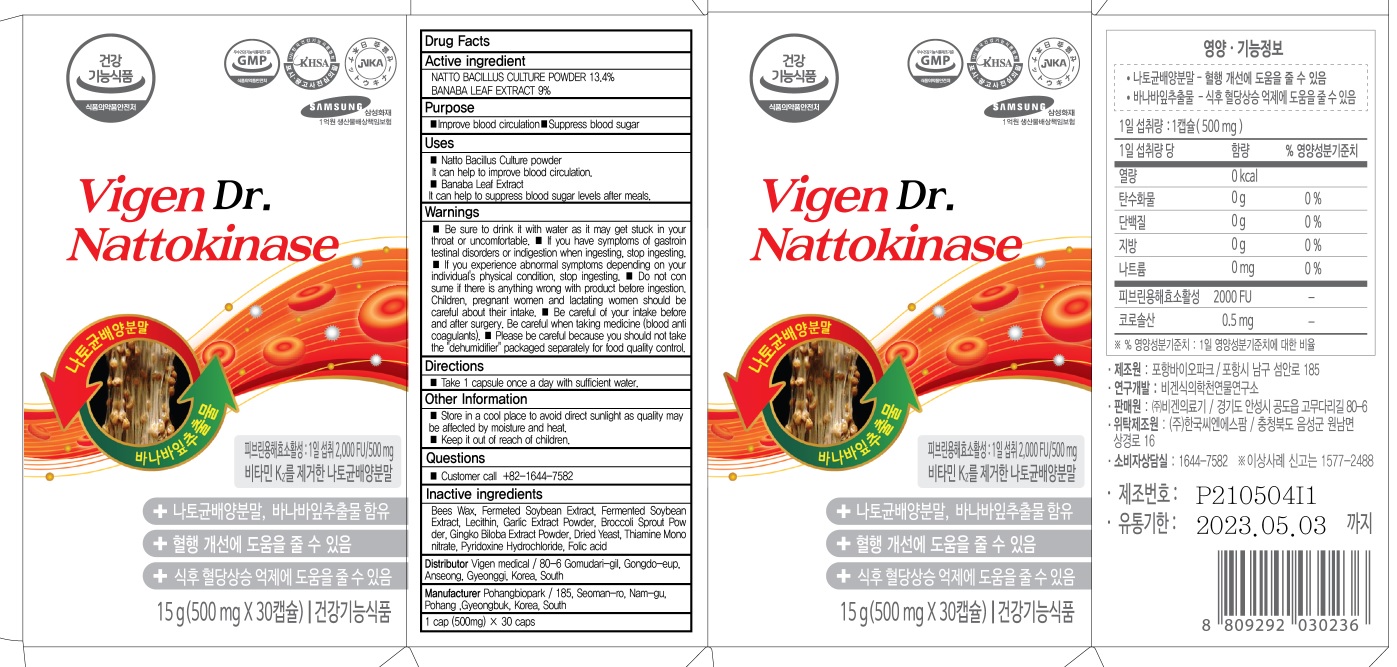
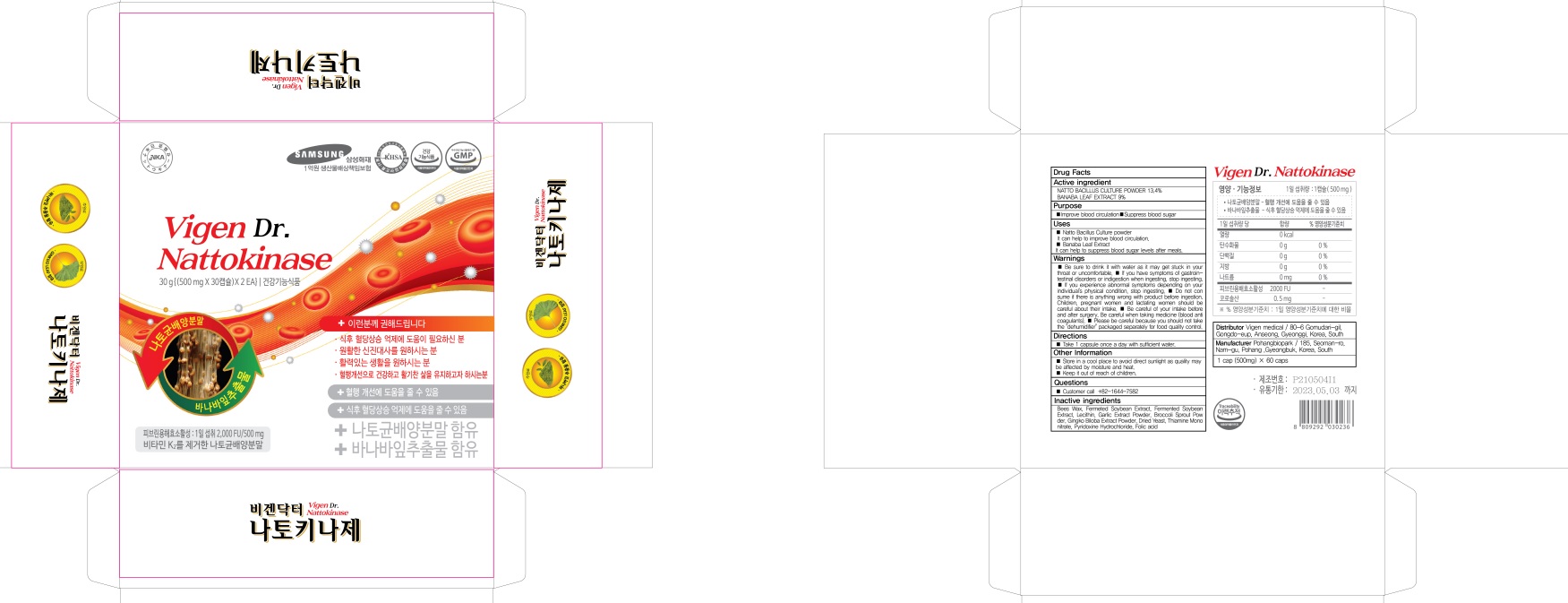
INACTIVE INGREDIENTS
Bees Wax, Fermeted Soybean Extract, Fermented Soybean Extract, Lecithin, Garlic Extract Powder, Broccoli Sprout Powder, Gingko Biloba Extract Powder, Dried Yeast, Thiamine Mononitrate, Pyridoxine Hydrochloride, Folic acid
WARNINGS
■ Be sure to drink it with water as it may get stuck in your throat or uncomfortable.
■ If you have symptoms of gastrointestinal disorders or indigestion when ingesting, stop ingesting.
■ If you experience abnormal symptoms depending on your individual's physical condition, stop ingesting.
■ Do not consume if there is anything wrong with product before ingestion. Children, pregnant women and lactating women should be careful about their intake.
■ Be careful of your intake before and after surgery. Be careful when taking medicine (blood anticoagulants).
■ Please be careful because you should not take the "dehumidifier" packaged separately for food quality control.
Uses
■ Natto Bacillus Culture powder
It can help to improve blood circulation.
■ Banaba Leaf Extract
It can help to suppress blood sugar levels after meals.