Label: HYDRATE- calcium citrate, sodium citrate, unspecified form, sea salt, potassium chloride, dibasic potassium phosphate, coconut oil, sunflower oil, avocado oil, aloe vera leaf, althaea officinalis root, ulmus rubra bark, and streptococcus salivarius powder, for solution
- NHRIC Code(s): 69833-020-30
- Packager: Spring Hill Therapeutics LLC
- Category: DIETARY SUPPLEMENT
- DEA Schedule: None
- Marketing Status: Dietary Supplement
Drug Label Information
Updated August 31, 2020
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
STATEMENT OF IDENTITY
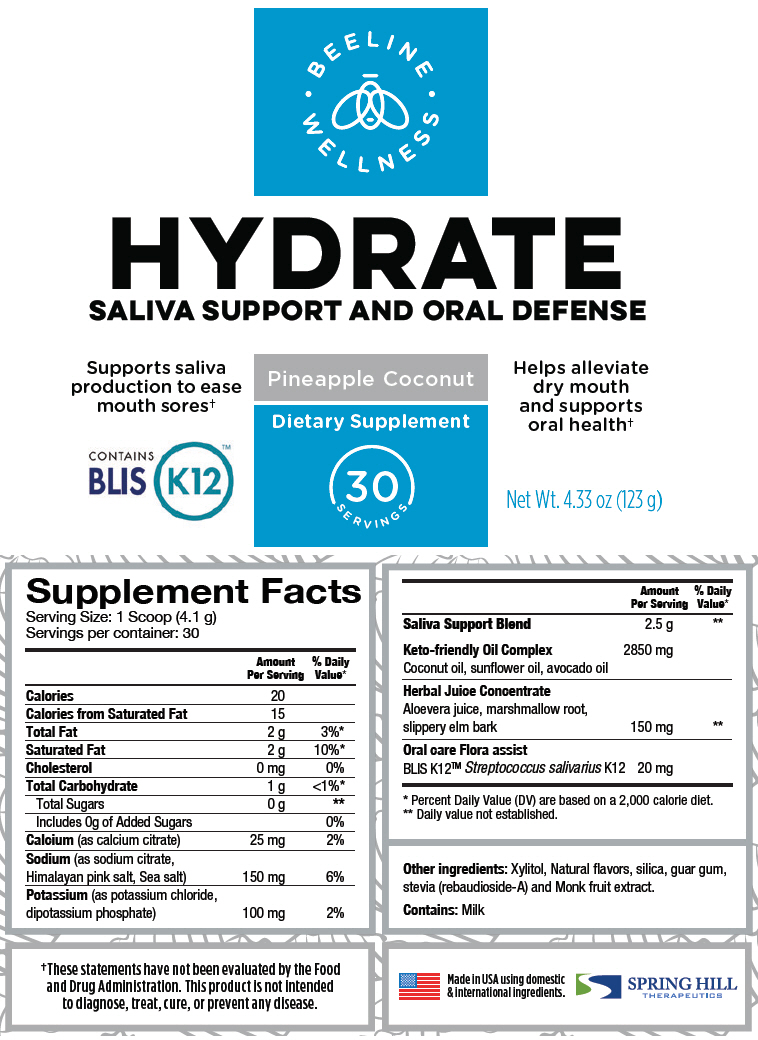
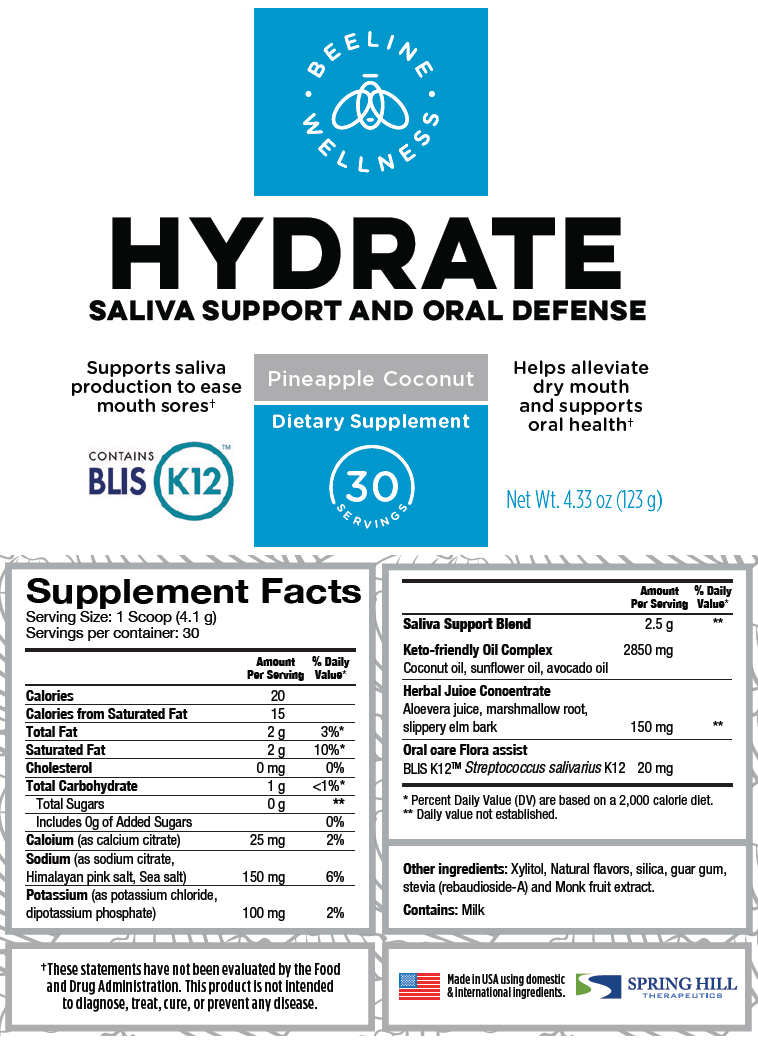
Supplement Facts Serving Size: 1 Scoop (4.1 g) Servings per container: 30 Amount Per Serving % Daily Value* Calories 20 Calories from Saturated Fat 15 Total Fat 2 g 3%* Saturated Fat 2 g 10%* Cholesterol 0 mg 0% Total Carbohydrate 1 g <1%* Total Sugars 0 g † Includes 0g of Added Sugars 0% Calcium (as calcium citrate) 25 mg 2% Sodium (as sodium citrate, Himalayan pink salt, Sea salt) 150 mg 6% Potassium (as potassium chloride, dipotassium phosphate) 100 mg 2% Saliva Support Blend 2.5 g † Keto-friendly Oil Complex 2850 mg Coconut oil, sunflower oil, avocado oil Herbal Juice Concentrate Aloevera juice, marshmallow root, slippery elm bark 150 mg † Oral care Flora assist BLIS K12™ Streptococcus salivarius K12 20 mg Other ingredients: Xylitol, Natural flavors, silica, guar gum, stevia (rebaudioside-A) and Monk fruit extract.
Contains: Milk
- HEALTH CLAIM
- PRINCIPAL DISPLAY PANEL - 123 g Canister Label
-
INGREDIENTS AND APPEARANCE
HYDRATE
calcium citrate, sodium citrate, unspecified form, sea salt, potassium chloride, dibasic potassium phosphate, coconut oil, sunflower oil, avocado oil, aloe vera leaf, althaea officinalis root, ulmus rubra bark, and streptococcus salivarius powder, for solutionProduct Information Product Type DIETARY SUPPLEMENT Item Code (Source) NHRIC:69833-020 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength calcium citrate (UNII: MLM29U2X85) (CALCIUM CATION - UNII:2M83C4R6ZB) CALCIUM CITRATE ANHYDROUS 25 mg in 4.1 g sodium citrate, unspecified form (UNII: 1Q73Q2JULR) (ANHYDROUS CITRIC ACID - UNII:XF417D3PSL) sodium citrate, unspecified form 150 mg in 4.1 g sea salt (UNII: 87GE52P74G) (sea salt - UNII:87GE52P74G) sea salt 150 mg in 4.1 g potassium chloride (UNII: 660YQ98I10) (POTASSIUM CATION - UNII:295O53K152) potassium chloride 100 mg in 4.1 g dibasic potassium phosphate (UNII: CI71S98N1Z) (PHOSPHATE ION - UNII:NK08V8K8HR) dibasic potassium phosphate 100 mg in 4.1 g Coconut oil (UNII: Q9L0O73W7L) (Coconut oil - UNII:Q9L0O73W7L) Coconut oil 2850 mg in 4.1 g sunflower oil (UNII: 3W1JG795YI) (sunflower oil - UNII:3W1JG795YI) sunflower oil 2850 mg in 4.1 g avocado oil (UNII: 6VNO72PFC1) (avocado oil - UNII:6VNO72PFC1) avocado oil 2850 mg in 4.1 g Aloe vera leaf (UNII: ZY81Z83H0X) (Aloe vera leaf - UNII:ZY81Z83H0X) Aloe vera leaf 150 mg in 4.1 g althaea officinalis root (UNII: TRW2FUF47H) (althaea officinalis root - UNII:TRW2FUF47H) althaea officinalis root 150 mg in 4.1 g ulmus rubra bark (UNII: 91QY4PXU8Q) (ulmus rubra bark - UNII:91QY4PXU8Q) ulmus rubra bark 150 mg in 4.1 g Streptococcus salivarius (UNII: NN52U3ZACZ) (Streptococcus salivarius - UNII:NN52U3ZACZ) Streptococcus salivarius 20 mg in 4.1 g Inactive Ingredients Ingredient Name Strength Xylitol (UNII: VCQ006KQ1E) silicon dioxide (UNII: ETJ7Z6XBU4) guar gum (UNII: E89I1637KE) rebaudioside A (UNII: B3FUD0528F) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NHRIC:69833-020-30 123 g in 1 CANISTER Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date DIETARY SUPPLEMENT 07/01/2020 Labeler - Spring Hill Therapeutics LLC (079813250)