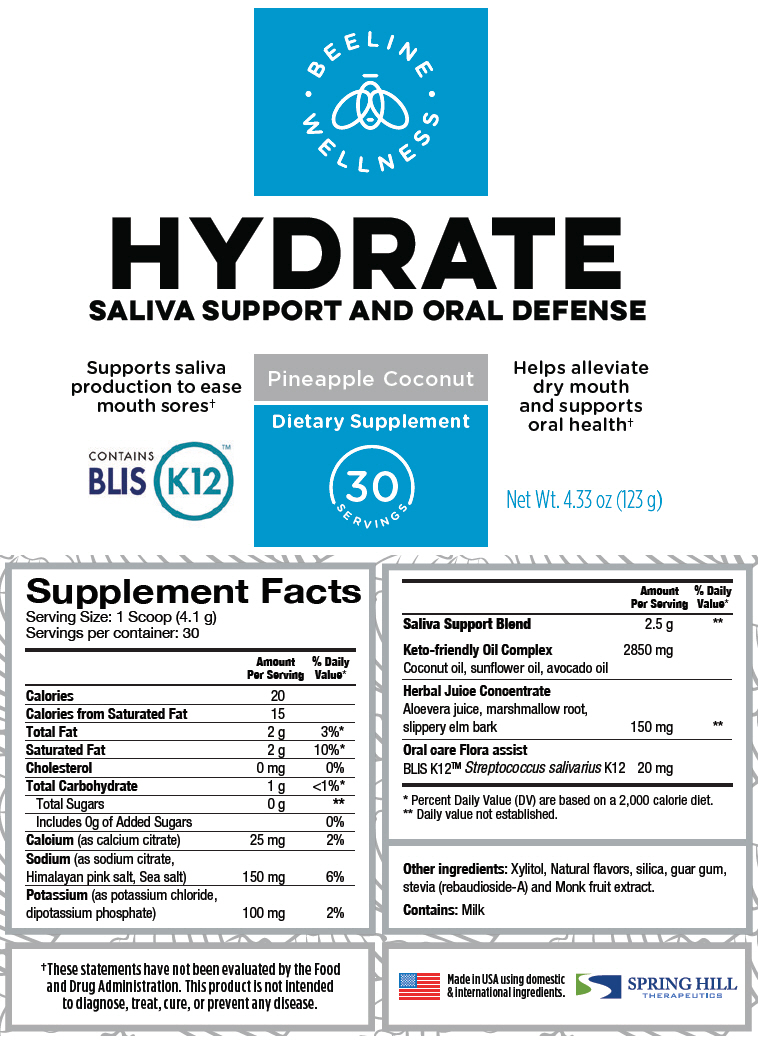
| Supplement Facts | ||
|---|---|---|
| Serving Size: 1 Scoop (4.1 g) | ||
| Servings per container: 30 | ||
| Amount Per Serving | % Daily Value* | |
| Calories | 20 | |
| Calories from Saturated Fat | 15 | |
| Total Fat | 2 g | 3%* |
| Saturated Fat | 2 g | 10%* |
| Cholesterol | 0 mg | 0% |
| Total Carbohydrate | 1 g | <1%* |
| Total Sugars | 0 g | † |
| Includes 0g of Added Sugars | 0% | |
| Calcium (as calcium citrate) | 25 mg | 2% |
| Sodium (as sodium citrate, Himalayan pink salt, Sea salt) | 150 mg | 6% |
| Potassium (as potassium chloride, dipotassium phosphate) | 100 mg | 2% |
| Saliva Support Blend | 2.5 g | † |
| Keto-friendly Oil Complex | 2850 mg | |
| Coconut oil, sunflower oil, avocado oil | ||
| Herbal Juice Concentrate | ||
| Aloevera juice, marshmallow root, slippery elm bark | 150 mg | † |
| Oral care Flora assist | ||
| BLIS K12™ Streptococcus salivarius K12 | 20 mg | |
Other ingredients: Xylitol, Natural flavors, silica, guar gum, stevia (rebaudioside-A) and Monk fruit extract.
Contains: Milk