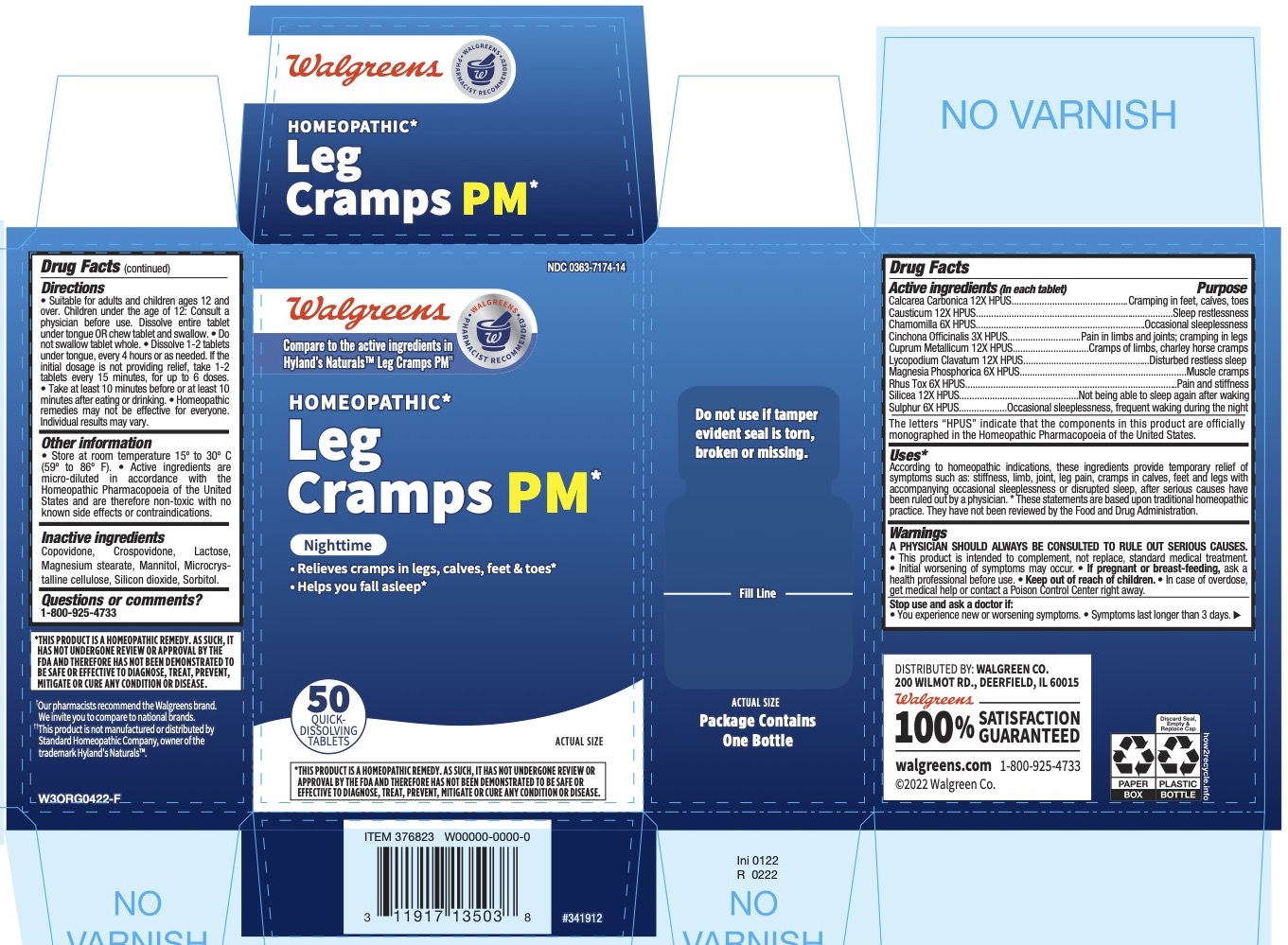
Label: LEG CRAMPS PM- oyster shell calcium carbonate, crude, causticum, matricaria recutita, cinchona officinalis bark, copper, lycopodium clavatum spore, magnesium phosphate, dibasic trihydrate, toxicodendron pubescens leaf, silicon dioxide, and sulfur tablet
- NDC Code(s): 0363-7174-14
- Packager: Walgreen Company
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved homeopathic
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated May 21, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- PURPOSE
-
Drug Facts
Active ingredients
Active ingredients Purpose Calcarea Carbonica 12X HPUS cramps in legs, calves, feet and toes Causticum 12X HPUS occasional sleeplessness Chamomilla 6X HPUS occasional sleeplessness Cinchona Officinalis 3X HPUS pain in limbs and joints; cramping in legs Cuprum Metallicum 12X HPUS cramps of limbs, charley horse cramps Lycopodium 12X HPUS disturbed, restless sleep Magnesia Phosphorica 6X HPUS muscle cramps Rhus Toxicodendron 6X HPUS pain and stiffness Silicea 12X HPUS not being able to sleep again after waking Sulphur 6X HPUS occasional sleeplessness, frequent waking during night “HPUS” indicates that the active ingredients are in the official Homeopathic Pharmacopœia of the United States.
-
Uses
■ According to homeopathic indications, these ingredients provide temporary relief of symptoms such as: stiffness, limb, joint, leg pain, cramps in calves, feet and legs with accompanying occasional sleeplessness or disrupted sleep, after serious causes have been ruled out by a physician. * These statements are based upon traditional homeopathic practice. They have not been reviewed by the Food and Drug Administration.
-
Warnings
A PHYSICIAN SHOULD ALWAYS BE CONSULTED TO RULE OUT SERIOUS CAUSES.
• This product is intended to complement, not replace, standard medical treatment. • Initial worsening of symptoms may occur.
-
Drug Facts(continued)
Directions
• Suitable for adults and children ages 12 and over. Children under the age of 12: Consult a physician before use. Dissolve entire tablet under tongue OR chew tablet and swallow. • Do not swallow tablet whole. • Dissolve 1-2 tablets under tongue, every 4 hours or as needed. If the initial dosage is not providing relief, take 1-2 tablets every 15 minutes, for up to 6 doses. • Take at least 10 minutes before or at least 10 minutes after eating or drinking. • Homeopathic remedies may not be effective for everyone. Individual results may vary.
- Other information
- Inactive ingredients
- SPL UNCLASSIFIED SECTION
- Questions or comments?
- Principal Display Panel - 50 Tablet Bottle Carton
-
INGREDIENTS AND APPEARANCE
LEG CRAMPS PM
oyster shell calcium carbonate, crude, causticum, matricaria recutita, cinchona officinalis bark, copper, lycopodium clavatum spore, magnesium phosphate, dibasic trihydrate, toxicodendron pubescens leaf, silicon dioxide, and sulfur tabletProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:0363-7174 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MATRICARIA RECUTITA (UNII: G0R4UBI2ZZ) (MATRICARIA RECUTITA - UNII:G0R4UBI2ZZ) MATRICARIA RECUTITA 6 [hp_X] LYCOPODIUM CLAVATUM SPORE (UNII: C88X29Y479) (LYCOPODIUM CLAVATUM SPORE - UNII:C88X29Y479) LYCOPODIUM CLAVATUM SPORE 12 [hp_X] CINCHONA OFFICINALIS BARK (UNII: S003A158SB) (CINCHONA OFFICINALIS BARK - UNII:S003A158SB) CINCHONA OFFICINALIS BARK 3 [hp_X] SILICON DIOXIDE (UNII: ETJ7Z6XBU4) (SILICON DIOXIDE - UNII:ETJ7Z6XBU4) SILICON DIOXIDE 12 [hp_X] SULFUR (UNII: 70FD1KFU70) (SULFUR - UNII:70FD1KFU70) SULFUR 6 [hp_X] COPPER (UNII: 789U1901C5) (COPPER - UNII:789U1901C5) COPPER 12 [hp_X] CAUSTICUM (UNII: DD5FO1WKFU) (CAUSTICUM - UNII:DD5FO1WKFU) CAUSTICUM 12 [hp_X] TOXICODENDRON PUBESCENS LEAF (UNII: 6IO182RP7A) (TOXICODENDRON PUBESCENS LEAF - UNII:6IO182RP7A) TOXICODENDRON PUBESCENS LEAF 6 [hp_X] MAGNESIUM PHOSPHATE, DIBASIC TRIHYDRATE (UNII: HF539G9L3Q) (MAGNESIUM CATION - UNII:T6V3LHY838) MAGNESIUM PHOSPHATE, DIBASIC TRIHYDRATE 6 [hp_X] OYSTER SHELL CALCIUM CARBONATE, CRUDE (UNII: 2E32821G6I) (OYSTER SHELL CALCIUM CARBONATE, CRUDE - UNII:2E32821G6I) OYSTER SHELL CALCIUM CARBONATE, CRUDE 12 [hp_X] Inactive Ingredients Ingredient Name Strength COPOVIDONE K25-31 (UNII: D9C330MD8B) MAGNESIUM STEARATE (UNII: 70097M6I30) MANNITOL (UNII: 3OWL53L36A) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) CROSPOVIDONE (UNII: 2S7830E561) SORBITOL (UNII: 506T60A25R) Product Characteristics Color white Score no score Shape ROUND Size 9mm Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0363-7174-14 1 in 1 CARTON 05/21/2023 1 50 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 05/21/2023 Labeler - Walgreen Company (008965063)