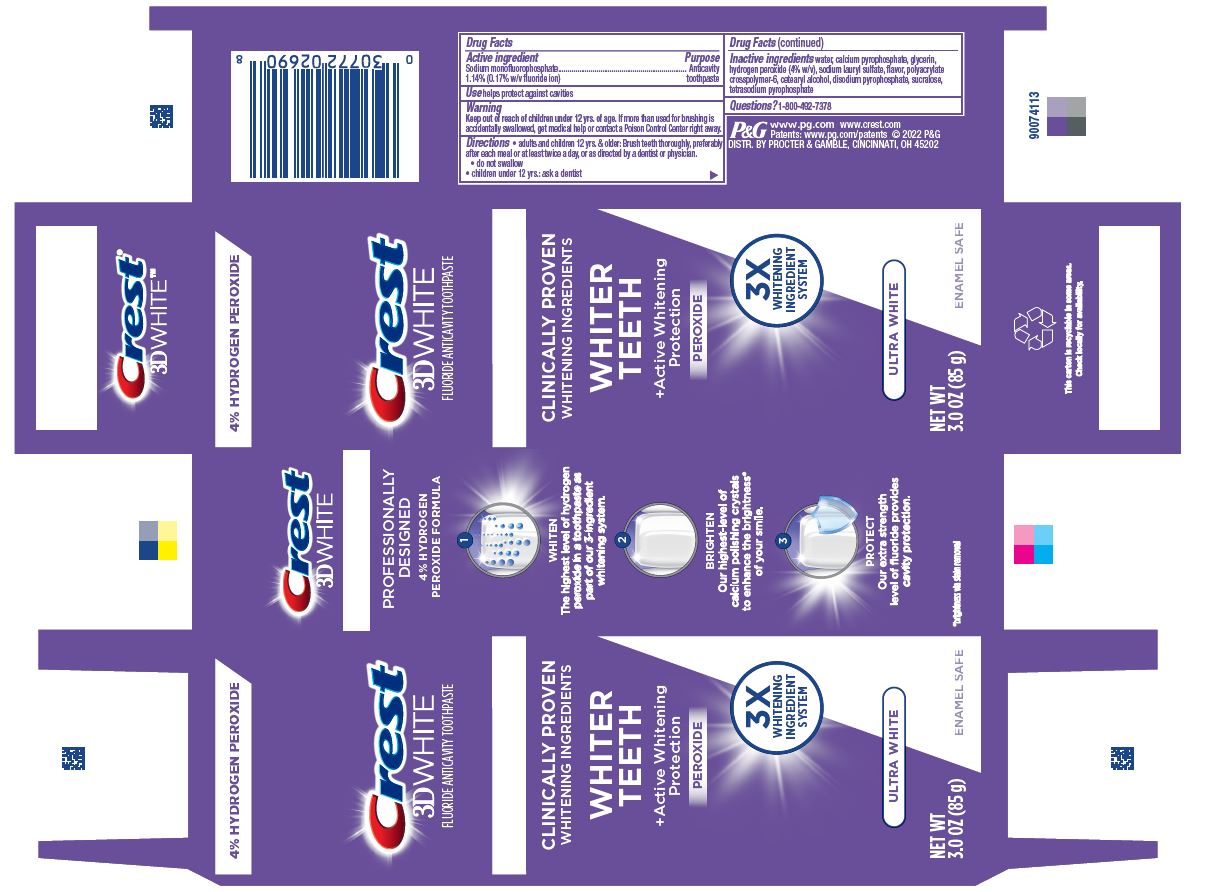
Label: CREST 3D WHITE PROFESSIONAL ULTRA WHITE- sodium monofluorophosphate paste, dentifrice
- NDC Code(s): 69423-874-30
- Packager: The Procter & Gamble Manufacturing Company
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated October 13, 2023
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Purpose
- Use
- WARNINGS
- Warning
- Directions
- Inactive ingredients
- Questions?
- SPL UNCLASSIFIED SECTION
- Crest 3D White Ultra White 85g tube in carton
-
INGREDIENTS AND APPEARANCE
CREST 3D WHITE PROFESSIONAL ULTRA WHITE
sodium monofluorophosphate paste, dentifriceProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69423-874 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SODIUM MONOFLUOROPHOSPHATE (UNII: C810JCZ56Q) (FLUORIDE ION - UNII:Q80VPU408O) FLUORIDE ION 1.7 mg in 1 g Inactive Ingredients Ingredient Name Strength CALCIUM PYROPHOSPHATE (UNII: X69NU20D19) GLYCERIN (UNII: PDC6A3C0OX) HYDROGEN PEROXIDE (UNII: BBX060AN9V) AMMONIUM ACRYLOYLDIMETHYLTAURATE, DIMETHYLACRYLAMIDE, LAURYL METHACRYLATE AND LAURETH-4 METHACRYLATE COPOLYMER, TRIMETHYLOLPROPANE TRIACRYLATE CROSSLINKED (45000 MPA.S) (UNII: Q7UI015FF9) SODIUM PYROPHOSPHATE (UNII: O352864B8Z) SODIUM LAURYL SULFATE (UNII: 368GB5141J) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) SODIUM ACID PYROPHOSPHATE (UNII: H5WVD9LZUD) WATER (UNII: 059QF0KO0R) SUCRALOSE (UNII: 96K6UQ3ZD4) Product Characteristics Color white Score Shape Size Flavor MINT Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69423-874-30 1 in 1 CARTON 01/01/2022 1 85 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M022 01/01/2022 Labeler - The Procter & Gamble Manufacturing Company (004238200)