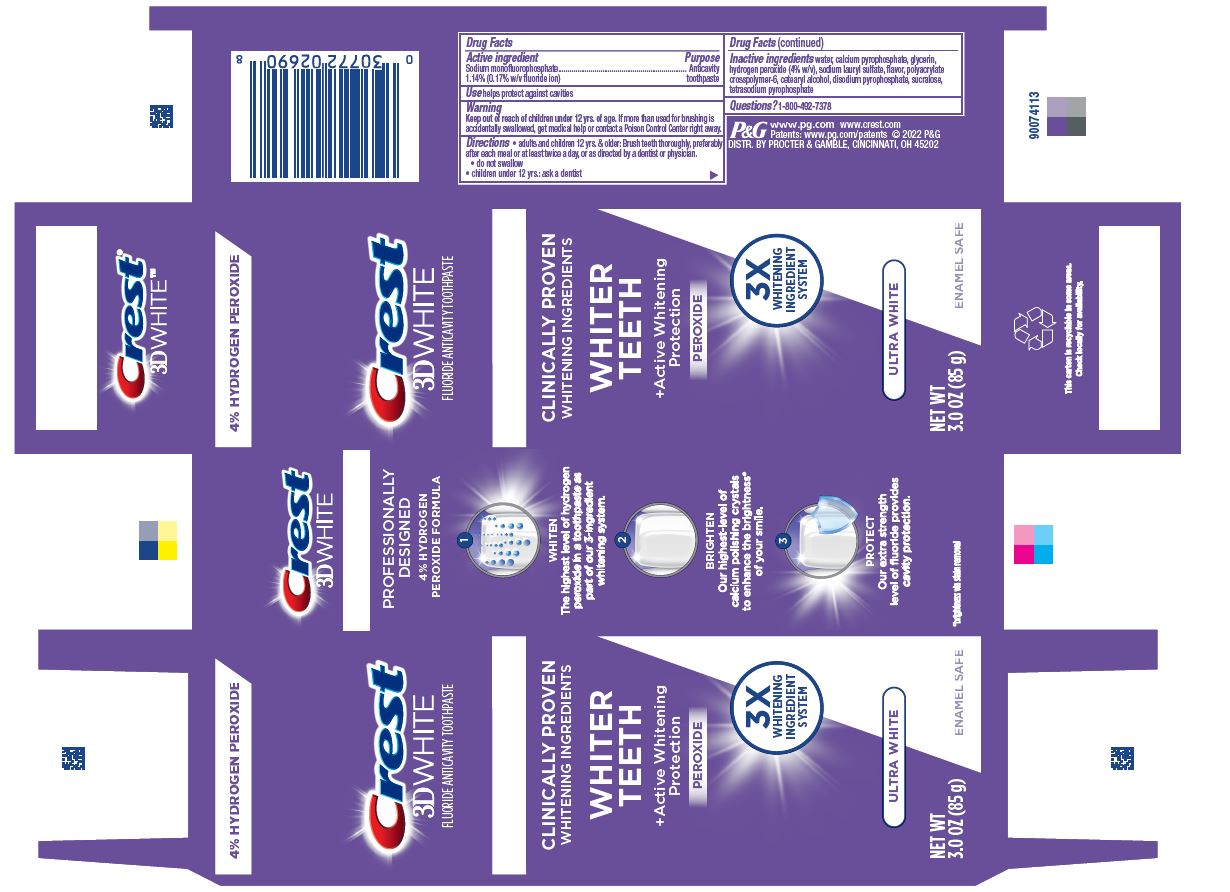
Drug Facts
Sodium monofluorophosphate 1.14%
(0.17% w/v fluoride ion)
Purpose
Anticavity toothpaste
Use
helps protect against cavities
Warning
Keep out of reach of children under 12 yrs. of age. If more than used for brushing is accidentally swallowed, get medical help or contact a Poison Control Center right away.
Directions
- adults and children 12 yrs. & older: Brush teeth thoroughly, preferably after each meal or at least twice a day, or as directed by a dentist or physician.
- do not swallow
- children under 12 yrs.: ask a dentist
Inactive ingredients
water, calcium pyrophosphate, glycerin, hydrogen peroxide (4% w/v), sodium lauryl sulfate, flavor, polyacrylate crosspolymer-6, cetearyl alcohol, disodium pyrophosphate, sucralose, tetrasodium pyrophosphate
Questions?
1-800-492-7378
DISTR. BY PROCTER & GAMBLE, CINCINNATI, OH 45202
Crest 3D White Ultra White 85g tube in carton
Crest
3D WHITE
FLUORIDE ANTICAVITY TOOTHPASTE
CLINICALLY PROVEN
WHITENING INGREDIENTS
WHITER TEETH
+Active Whitening
Protection
PEROXIDE
3X WHITENING INGREDIENT SYSTEM
ULTRA WHITE
NET WT 3.0 OZ (85 g)
ENAMEL SAFE