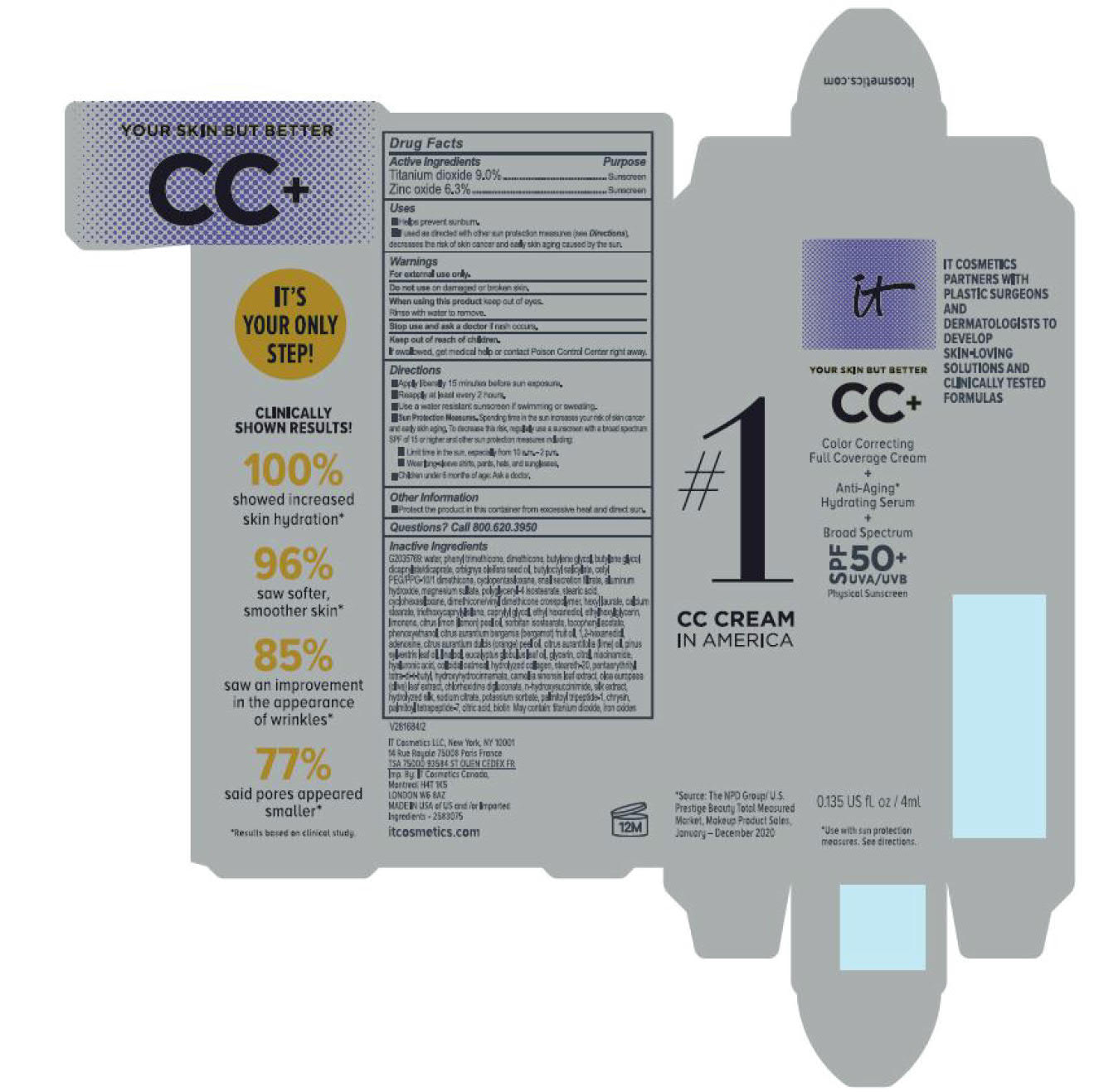
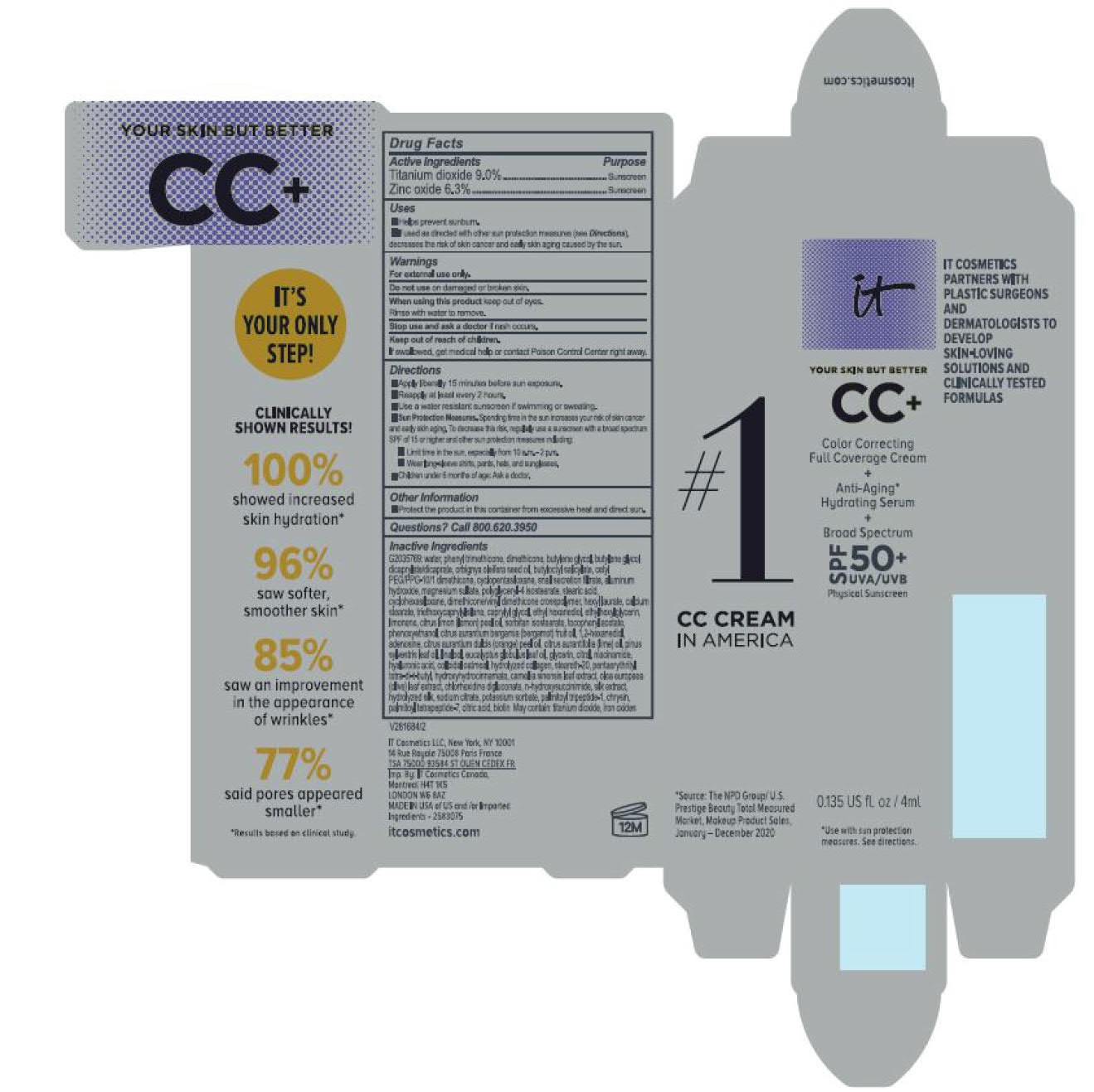
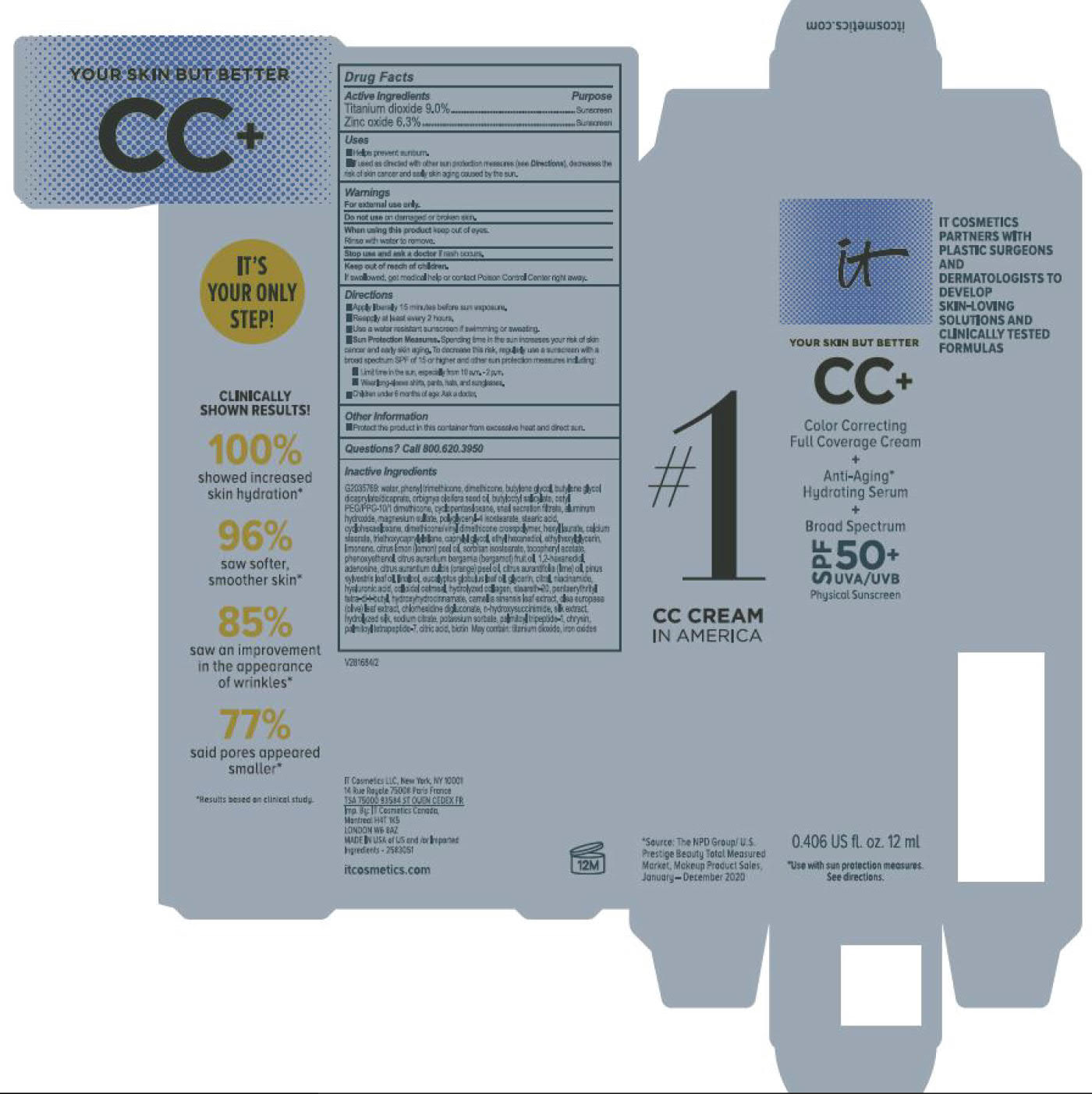
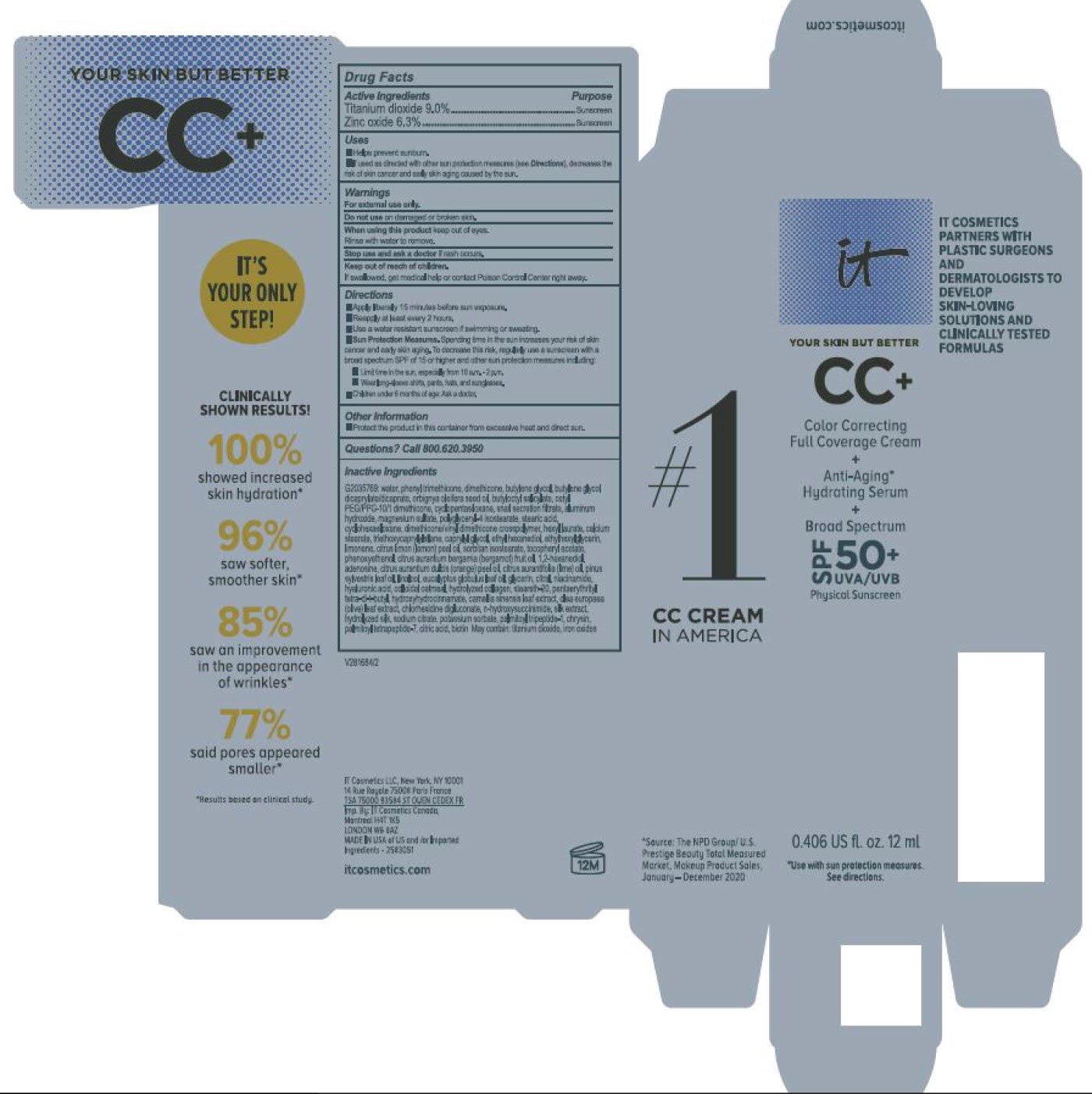
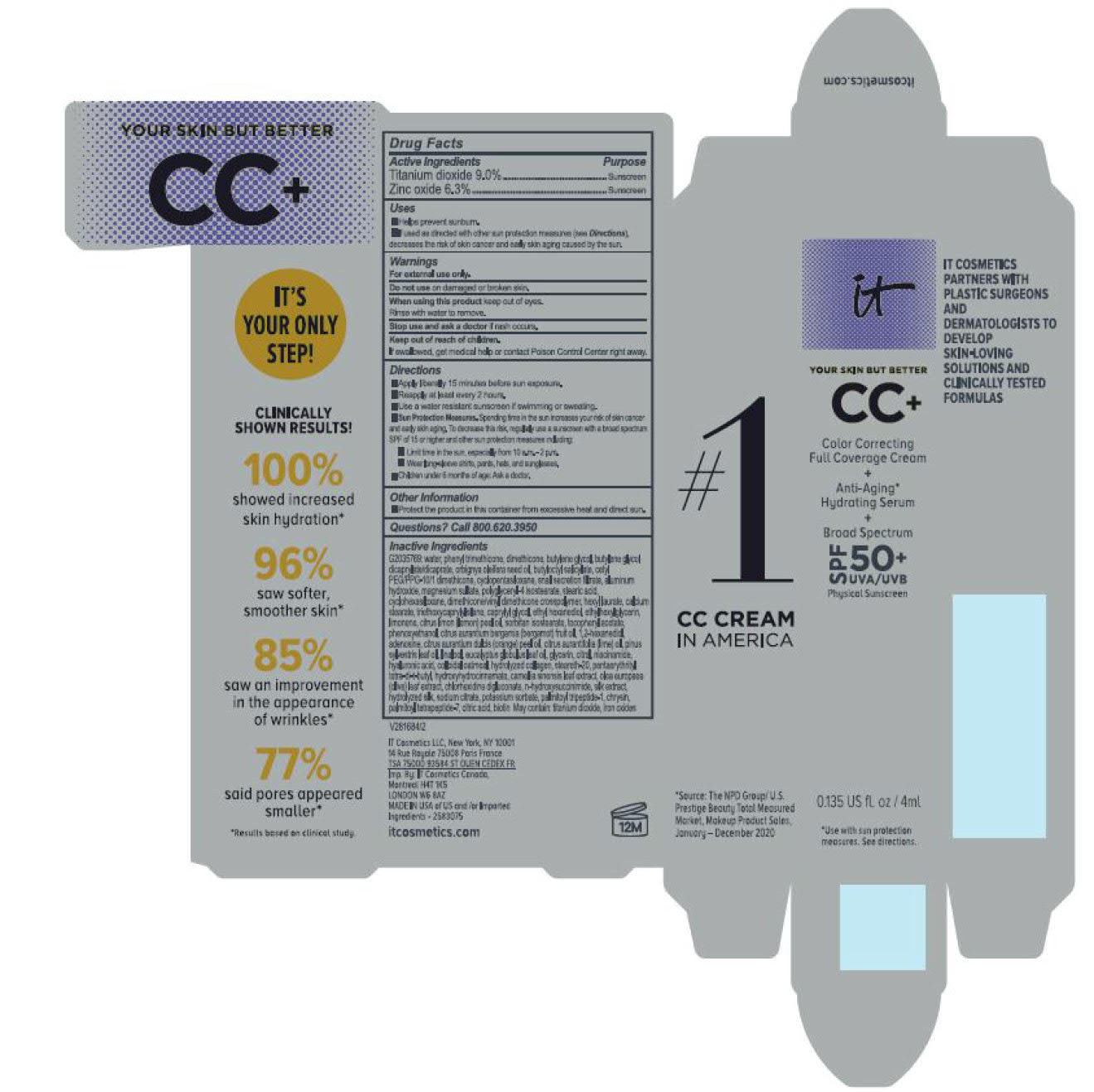
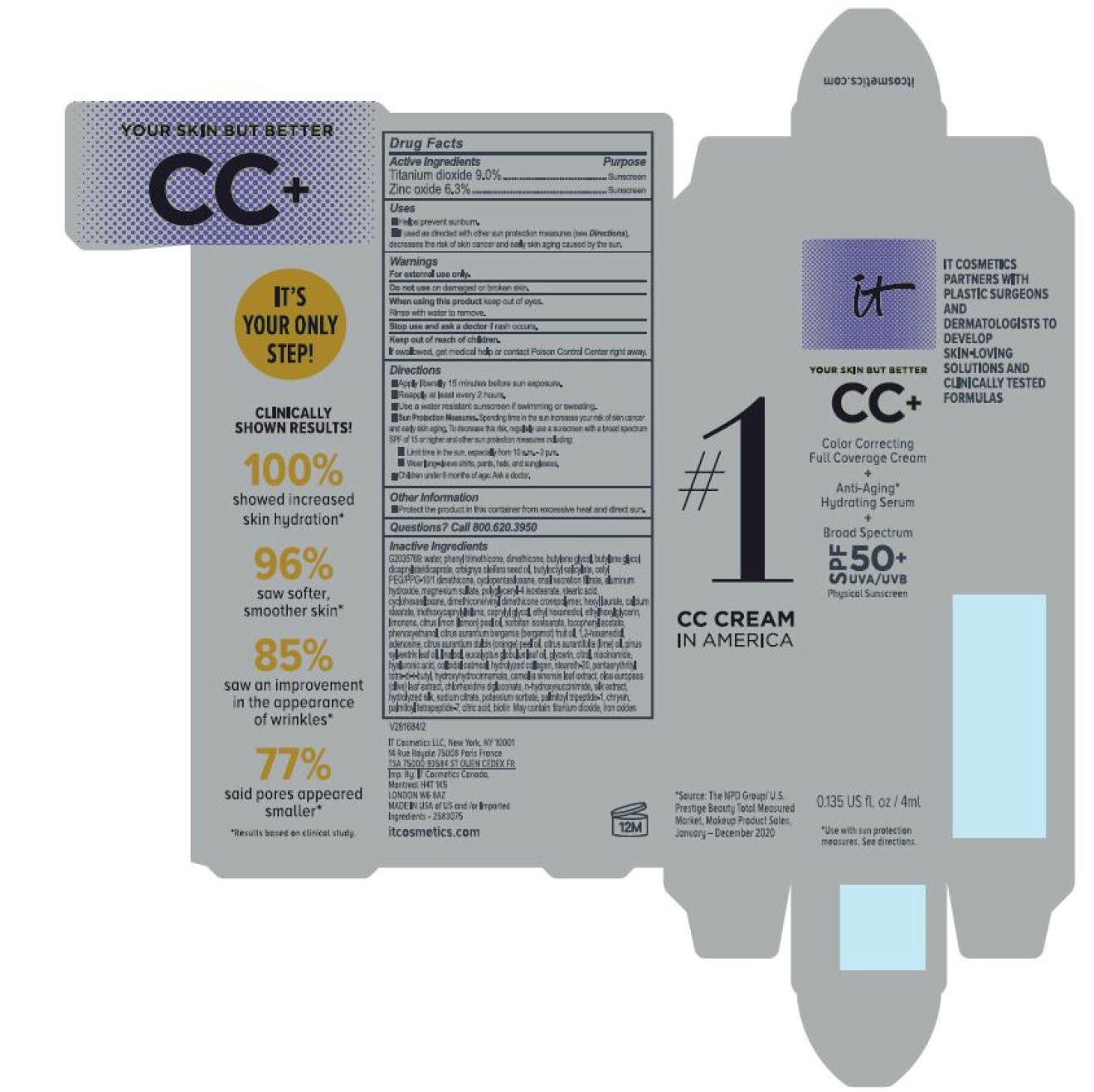
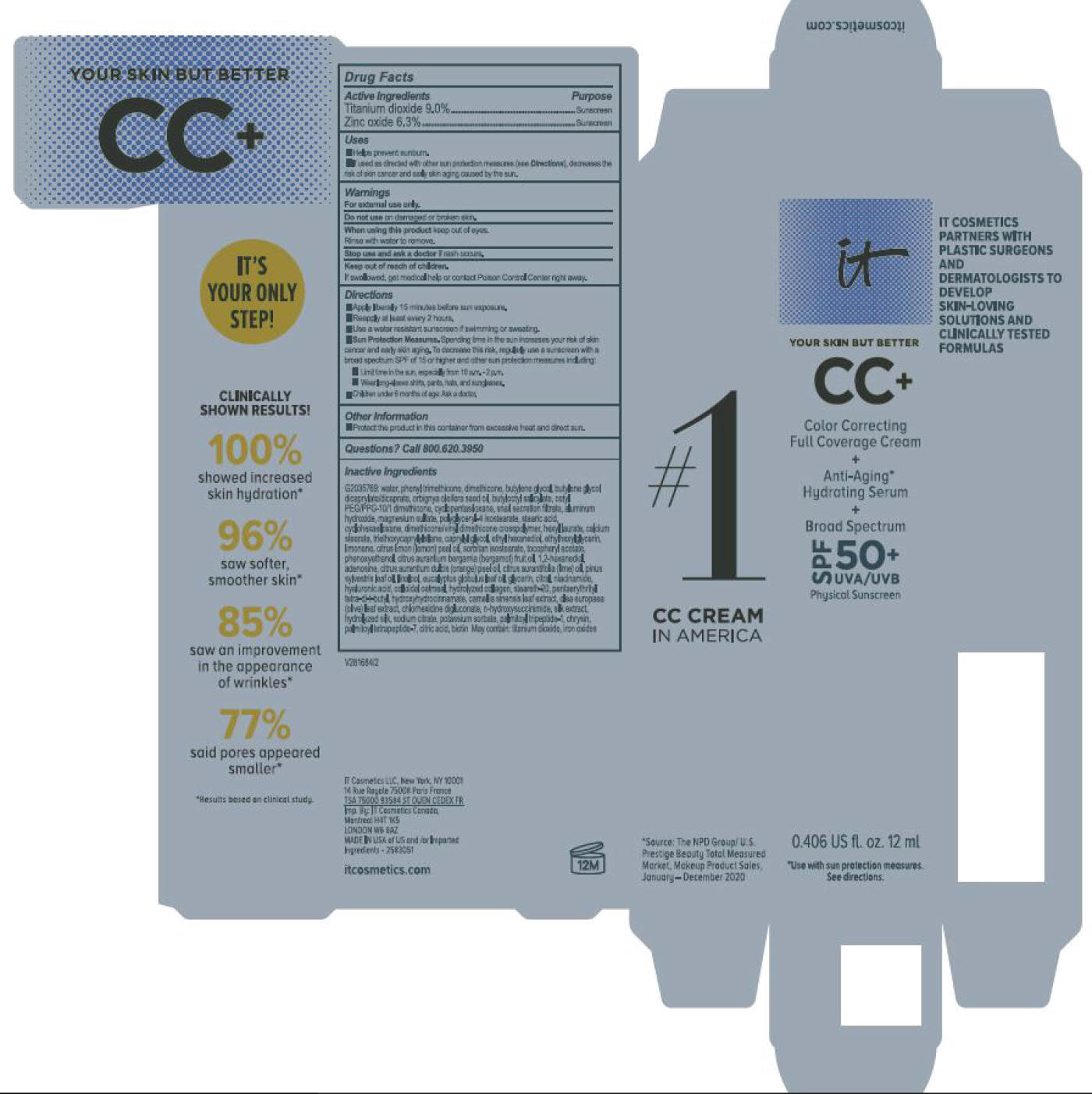
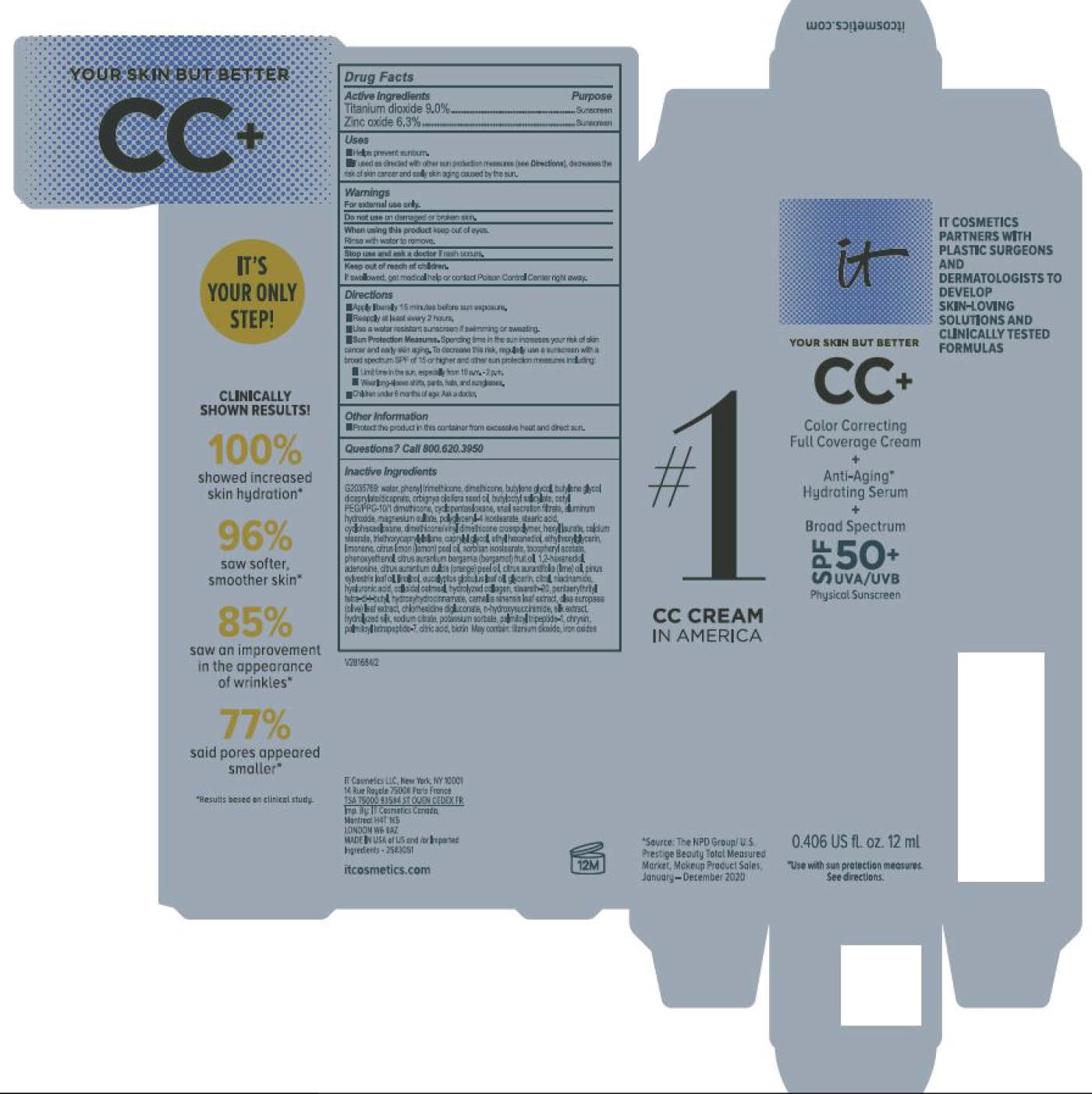
Label: ITC CC FULL COVERAGE CREAM NEUTRAL MEDIUM- titanium dioxide, zinc oxide cream
ITC CC FULL COVERAGE CREAM NEUTRAL TAN- titanium dioxide, zinc oxide cream
ITC CC FULL COVERAGE CREAM FAIR LIGHT- titanium dioxide, zinc oxide cream
ITC CC FULL COVERAGE CREAM LIGHT- titanium dioxide, zinc oxide cream
ITC CC FULL COVERAGE CREAM MEDIUM- titanium dioxide, zinc oxide cream
ITC CC FULL COVERAGE CREAM MEDIUM TAN- titanium dioxide, zinc oxide cream
ITC CC FULL COVERAGE CREAM TAN- titanium dioxide, zinc oxide cream
ITC CC FULL COVERAGE CREAM RICH HONEY- titanium dioxide, zinc oxide cream
ITC CC FULL COVERAGE CREAM FAIR- titanium dioxide, zinc oxide cream
ITC CC FULL COVERAGE CREAM LIGHT MEDIUM- titanium dioxide, zinc oxide cream
ITC CC FULL COVERAGE CREAM RICH- titanium dioxide, zinc oxide cream
-
NDC Code(s):
11090-103-01,
11090-103-02,
11090-103-03,
11090-103-04, view more11090-104-01, 11090-104-02, 11090-104-03, 11090-105-01, 11090-105-02, 11090-105-03, 11090-105-04, 11090-106-01, 11090-106-02, 11090-106-03, 11090-107-01, 11090-107-02, 11090-107-03, 11090-107-04, 11090-108-01, 11090-108-02, 11090-108-03, 11090-109-01, 11090-109-02, 11090-109-03, 11090-109-04, 11090-110-01, 11090-110-02, 11090-110-03, 11090-111-01, 11090-111-02, 11090-111-03, 11090-111-04, 11090-112-01, 11090-112-02, 11090-112-03, 11090-112-04, 11090-113-01, 11090-113-02, 11090-113-03
- Packager: Beauty Manufacturing Solutions Corp.
- Category: HUMAN OTC DRUG LABEL
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated February 11, 2022
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
- WARNINGS
- DO NOT USE
- ASK DOCTOR
- KEEP OUT OF REACH OF CHILDREN
-
DOSAGE & ADMINISTRATION
For sunscreen use:
● apply liberally 15 minutes before sun exposure
● reapply at least every 2 hours
● use a water resistant sunscreen if swimming or sweating
● Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including:
● limit time in the sun, especially from 10 a.m. – 2 p.m.
● wear long-sleeved shirts, pants, hats, and sunglasses
● children under 6 months of age: Ask a doctor - STORAGE AND HANDLING
- QUESTIONS
-
INACTIVE INGREDIENT
water, snail secretion filtrate, phenyl trimethicone, dimethicone, butylene glycol, butylene glycol dicaprylated/dicaprate, orbignya oleifera seed oil, butyloctyl salicylate, cetyl PEG/PPG-10/1 dimethiocone, cyclopentasiloxane, cyclohexasiloxane, magnesium sulfate, polyglyceryl-4 isostearate, dimethicone/vinyl dimethicone crosspolymer, aluminum hydroxide, hexyl laurate, stearic acid, calcium stearate, caprylyl glycol, triethoxycaprylylsilane, ethylhexylglycerin, citrus medica limonum (lemon) peel oil, tocopheryl acetate, sorbitan isostearate, phenoxyethanol, citrus aurantium bergamia (bergamot) fruit oil, 1,2-hexanediol, disodium EDTA, citrus aurantium dulcis (orange) peel oil, cirus aurantifolia (lime) oil vitis vinifera (grape) seed oil, punica gratissiima (avocado) oil, niacinamide, citrus grandis (grapefuit) peel oil, chloesterol, anthemis b=nobilis flower water, lactobacillius/honeysuckle flowr/locorice root/morus alba root/pueraria lobata root/schizandra chinensis fruit/scutellaria baicalensis root/sophora japonica flower extract ferment filtrate, perfluorohexane, olea europaea (olive) leaf extract, glycerin, eucalyptus globulus leaf oil, camellia sinensis leaf extract, chrysanthemium indicum flower extract, pueraria lobata root extract, perfluorodecalin, morus alba gruit extract, magnolia kobus bark extract, glycine soja (soybean) sprout extract, diospyros kaki leaf extract, cinnamomum cassia bark extract, artemisia princeps leaf extract, epntafluoropropane, curcuma longa (turmeric) root extract, steareth-20, hydrolyzed silk, hydrolyzed hyaluronic acid, colloidal oatmeal, citric acid, sodium benzoate, potassum sorbate, aloe barbadensis leaf extract, n-hydroxysuccinimide, hydrolyzed collagen, caprylhydroxamic acid, tocopherol, thiamine HCL, riboflavin, retinyl palmitate, pantothenic acid, palmitoyl oligopeptide, nicin, folic acid, chrysin, carnitine HCL, biotin, ascorbic acid, palmitoyl tetrapeptide-7, chlorhexidine digluconate; may contain: iron oxides
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
ITC CC FULL COVERAGE CREAM NEUTRAL MEDIUM
titanium dioxide, zinc oxide creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:11090-108 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 90 mg in 1 mL ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 63 mg in 1 mL Inactive Ingredients Ingredient Name Strength ALPHA-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) GLYCERIN (UNII: PDC6A3C0OX) 1,2-HEXANEDIOL (UNII: TR046Y3K1G) HEXYL LAURATE (UNII: 4CG9F9W01Q) STEARIC ACID (UNII: 4ELV7Z65AP) BOMBYX MORI FIBER (UNII: 6LK42KUV6W) DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER (SOFT PARTICLE) (UNII: 9E4CO0W6C5) PHENOXYETHANOL (UNII: HIE492ZZ3T) LEMON OIL (UNII: I9GRO824LL) STEARETH-20 (UNII: L0Q8IK9E08) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) FERRIC OXIDE RED (UNII: 1K09F3G675) PINE NEEDLE OIL (PINUS SYLVESTRIS) (UNII: 5EXL5H740Y) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) NIACINAMIDE (UNII: 25X51I8RD4) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) EUCALYPTUS OIL (UNII: 2R04ONI662) CHRYSIN (UNII: 3CN01F5ZJ5) BUTYLENE GLYCOL DICAPRYLATE/DICAPRATE (UNII: 75D21FL1PI) CALCIUM STEARATE (UNII: 776XM7047L) BABASSU OIL (UNII: 8QSB4M5477) DIMETHICONE (UNII: 92RU3N3Y1O) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) ADENOSINE (UNII: K72T3FS567) GREEN TEA LEAF (UNII: W2ZU1RY8B0) CYCLOMETHICONE 6 (UNII: XHK3U310BA) PHENYL TRIMETHICONE (UNII: DR0K5NOJ4R) MAGNESIUM SULFATE HEPTAHYDRATE (UNII: SK47B8698T) LIME OIL (UNII: UZH29XGA8G) FERROSOFERRIC OXIDE (UNII: XM0M87F357) CETYL PEG/PPG-10/1 DIMETHICONE (HLB 5) (UNII: 035JKJ76MT) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) POLYGLYCERYL-4 ISOSTEARATE (UNII: 820DPX33S7) OATMEAL (UNII: 8PI54V663Y) BERGAMOT OIL (UNII: 39W1PKE3JI) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) CAPRYLYL GLYCOL (UNII: 00YIU5438U) SORBITAN ISOSTEARATE (UNII: 01S2G2C1E4) WATER (UNII: 059QF0KO0R) GELATIN HYDROLYSATE (PORCINE SKIN, MW 3000) (UNII: 0K9R94573C) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) ORANGE OIL (UNII: AKN3KSD11B) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) OLEA EUROPAEA LEAF (UNII: MJ95C3OH47) N-HYDROXYSUCCINIMIDE (UNII: MJE3791M4T) CHLORHEXIDINE GLUCONATE (UNII: MOR84MUD8E) PALMITOYL TETRAPEPTIDE-7 (UNII: Q41S464P1R) PALMITOYL TRIPEPTIDE-1 (UNII: RV743D216M) HYALURONIC ACID (UNII: S270N0TRQY) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:11090-108-01 1 in 1 CARTON 03/14/2018 1 12 mL in 1 TUBE; Type 0: Not a Combination Product 2 NDC:11090-108-03 1 in 1 CARTON 03/14/2018 2 75 mL in 1 TUBE; Type 0: Not a Combination Product 3 NDC:11090-108-02 1 in 1 CARTON 03/14/2018 3 32 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part352 03/14/2018 ITC CC FULL COVERAGE CREAM NEUTRAL TAN
titanium dioxide, zinc oxide creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:11090-110 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 90 mg in 1 mL ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 63 mg in 1 mL Inactive Ingredients Ingredient Name Strength ALPHA-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) GLYCERIN (UNII: PDC6A3C0OX) 1,2-HEXANEDIOL (UNII: TR046Y3K1G) HEXYL LAURATE (UNII: 4CG9F9W01Q) STEARIC ACID (UNII: 4ELV7Z65AP) BOMBYX MORI FIBER (UNII: 6LK42KUV6W) DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER (SOFT PARTICLE) (UNII: 9E4CO0W6C5) PHENOXYETHANOL (UNII: HIE492ZZ3T) LEMON OIL (UNII: I9GRO824LL) STEARETH-20 (UNII: L0Q8IK9E08) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) FERRIC OXIDE RED (UNII: 1K09F3G675) PINE NEEDLE OIL (PINUS SYLVESTRIS) (UNII: 5EXL5H740Y) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) NIACINAMIDE (UNII: 25X51I8RD4) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) EUCALYPTUS OIL (UNII: 2R04ONI662) CHRYSIN (UNII: 3CN01F5ZJ5) BUTYLENE GLYCOL DICAPRYLATE/DICAPRATE (UNII: 75D21FL1PI) CALCIUM STEARATE (UNII: 776XM7047L) BABASSU OIL (UNII: 8QSB4M5477) DIMETHICONE (UNII: 92RU3N3Y1O) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) ADENOSINE (UNII: K72T3FS567) GREEN TEA LEAF (UNII: W2ZU1RY8B0) CYCLOMETHICONE 6 (UNII: XHK3U310BA) PHENYL TRIMETHICONE (UNII: DR0K5NOJ4R) MAGNESIUM SULFATE HEPTAHYDRATE (UNII: SK47B8698T) LIME OIL (UNII: UZH29XGA8G) FERROSOFERRIC OXIDE (UNII: XM0M87F357) CETYL PEG/PPG-10/1 DIMETHICONE (HLB 5) (UNII: 035JKJ76MT) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) POLYGLYCERYL-4 ISOSTEARATE (UNII: 820DPX33S7) OATMEAL (UNII: 8PI54V663Y) BERGAMOT OIL (UNII: 39W1PKE3JI) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) CAPRYLYL GLYCOL (UNII: 00YIU5438U) SORBITAN ISOSTEARATE (UNII: 01S2G2C1E4) WATER (UNII: 059QF0KO0R) GELATIN HYDROLYSATE (PORCINE SKIN, MW 3000) (UNII: 0K9R94573C) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) ORANGE OIL (UNII: AKN3KSD11B) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) OLEA EUROPAEA LEAF (UNII: MJ95C3OH47) N-HYDROXYSUCCINIMIDE (UNII: MJE3791M4T) CHLORHEXIDINE GLUCONATE (UNII: MOR84MUD8E) PALMITOYL TETRAPEPTIDE-7 (UNII: Q41S464P1R) PALMITOYL TRIPEPTIDE-1 (UNII: RV743D216M) HYALURONIC ACID (UNII: S270N0TRQY) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:11090-110-01 1 in 1 CARTON 03/14/2018 1 12 mL in 1 TUBE; Type 0: Not a Combination Product 2 NDC:11090-110-03 1 in 1 CARTON 03/14/2018 2 75 mL in 1 TUBE; Type 0: Not a Combination Product 3 NDC:11090-110-02 1 in 1 CARTON 03/14/2018 3 32 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part352 03/14/2018 ITC CC FULL COVERAGE CREAM FAIR LIGHT
titanium dioxide, zinc oxide creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:11090-104 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 90 mg in 1 mL ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 63 mg in 1 mL Inactive Ingredients Ingredient Name Strength ALPHA-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) GLYCERIN (UNII: PDC6A3C0OX) 1,2-HEXANEDIOL (UNII: TR046Y3K1G) HEXYL LAURATE (UNII: 4CG9F9W01Q) STEARIC ACID (UNII: 4ELV7Z65AP) BOMBYX MORI FIBER (UNII: 6LK42KUV6W) DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER (SOFT PARTICLE) (UNII: 9E4CO0W6C5) PHENOXYETHANOL (UNII: HIE492ZZ3T) LEMON OIL (UNII: I9GRO824LL) STEARETH-20 (UNII: L0Q8IK9E08) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) FERRIC OXIDE RED (UNII: 1K09F3G675) PINE NEEDLE OIL (PINUS SYLVESTRIS) (UNII: 5EXL5H740Y) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) NIACINAMIDE (UNII: 25X51I8RD4) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) EUCALYPTUS OIL (UNII: 2R04ONI662) CHRYSIN (UNII: 3CN01F5ZJ5) BUTYLENE GLYCOL DICAPRYLATE/DICAPRATE (UNII: 75D21FL1PI) CALCIUM STEARATE (UNII: 776XM7047L) BABASSU OIL (UNII: 8QSB4M5477) DIMETHICONE (UNII: 92RU3N3Y1O) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) ADENOSINE (UNII: K72T3FS567) GREEN TEA LEAF (UNII: W2ZU1RY8B0) CYCLOMETHICONE 6 (UNII: XHK3U310BA) PHENYL TRIMETHICONE (UNII: DR0K5NOJ4R) MAGNESIUM SULFATE HEPTAHYDRATE (UNII: SK47B8698T) LIME OIL (UNII: UZH29XGA8G) FERROSOFERRIC OXIDE (UNII: XM0M87F357) CETYL PEG/PPG-10/1 DIMETHICONE (HLB 5) (UNII: 035JKJ76MT) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) POLYGLYCERYL-4 ISOSTEARATE (UNII: 820DPX33S7) OATMEAL (UNII: 8PI54V663Y) BERGAMOT OIL (UNII: 39W1PKE3JI) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) CAPRYLYL GLYCOL (UNII: 00YIU5438U) SORBITAN ISOSTEARATE (UNII: 01S2G2C1E4) WATER (UNII: 059QF0KO0R) GELATIN HYDROLYSATE (PORCINE SKIN, MW 3000) (UNII: 0K9R94573C) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) ORANGE OIL (UNII: AKN3KSD11B) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) OLEA EUROPAEA LEAF (UNII: MJ95C3OH47) N-HYDROXYSUCCINIMIDE (UNII: MJE3791M4T) CHLORHEXIDINE GLUCONATE (UNII: MOR84MUD8E) PALMITOYL TETRAPEPTIDE-7 (UNII: Q41S464P1R) PALMITOYL TRIPEPTIDE-1 (UNII: RV743D216M) HYALURONIC ACID (UNII: S270N0TRQY) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:11090-104-01 1 in 1 CARTON 03/14/2018 1 12 mL in 1 TUBE; Type 0: Not a Combination Product 2 NDC:11090-104-03 1 in 1 CARTON 03/14/2018 2 75 mL in 1 TUBE; Type 0: Not a Combination Product 3 NDC:11090-104-02 1 in 1 CARTON 03/14/2018 3 32 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part352 03/14/2018 ITC CC FULL COVERAGE CREAM LIGHT
titanium dioxide, zinc oxide creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:11090-105 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 90 mg in 1 mL ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 63 mg in 1 mL Inactive Ingredients Ingredient Name Strength ALPHA-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) GLYCERIN (UNII: PDC6A3C0OX) 1,2-HEXANEDIOL (UNII: TR046Y3K1G) HEXYL LAURATE (UNII: 4CG9F9W01Q) STEARIC ACID (UNII: 4ELV7Z65AP) BOMBYX MORI FIBER (UNII: 6LK42KUV6W) DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER (SOFT PARTICLE) (UNII: 9E4CO0W6C5) PHENOXYETHANOL (UNII: HIE492ZZ3T) LEMON OIL (UNII: I9GRO824LL) STEARETH-20 (UNII: L0Q8IK9E08) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) FERRIC OXIDE RED (UNII: 1K09F3G675) PINE NEEDLE OIL (PINUS SYLVESTRIS) (UNII: 5EXL5H740Y) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) NIACINAMIDE (UNII: 25X51I8RD4) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) EUCALYPTUS OIL (UNII: 2R04ONI662) CHRYSIN (UNII: 3CN01F5ZJ5) BUTYLENE GLYCOL DICAPRYLATE/DICAPRATE (UNII: 75D21FL1PI) CALCIUM STEARATE (UNII: 776XM7047L) BABASSU OIL (UNII: 8QSB4M5477) DIMETHICONE (UNII: 92RU3N3Y1O) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) ADENOSINE (UNII: K72T3FS567) GREEN TEA LEAF (UNII: W2ZU1RY8B0) CYCLOMETHICONE 6 (UNII: XHK3U310BA) PHENYL TRIMETHICONE (UNII: DR0K5NOJ4R) MAGNESIUM SULFATE HEPTAHYDRATE (UNII: SK47B8698T) LIME OIL (UNII: UZH29XGA8G) FERROSOFERRIC OXIDE (UNII: XM0M87F357) CETYL PEG/PPG-10/1 DIMETHICONE (HLB 5) (UNII: 035JKJ76MT) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) POLYGLYCERYL-4 ISOSTEARATE (UNII: 820DPX33S7) OATMEAL (UNII: 8PI54V663Y) BERGAMOT OIL (UNII: 39W1PKE3JI) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) CAPRYLYL GLYCOL (UNII: 00YIU5438U) SORBITAN ISOSTEARATE (UNII: 01S2G2C1E4) WATER (UNII: 059QF0KO0R) GELATIN HYDROLYSATE (PORCINE SKIN, MW 3000) (UNII: 0K9R94573C) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) ORANGE OIL (UNII: AKN3KSD11B) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) OLEA EUROPAEA LEAF (UNII: MJ95C3OH47) N-HYDROXYSUCCINIMIDE (UNII: MJE3791M4T) CHLORHEXIDINE GLUCONATE (UNII: MOR84MUD8E) PALMITOYL TETRAPEPTIDE-7 (UNII: Q41S464P1R) PALMITOYL TRIPEPTIDE-1 (UNII: RV743D216M) HYALURONIC ACID (UNII: S270N0TRQY) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:11090-105-01 1 in 1 CARTON 03/14/2018 1 4 mL in 1 TUBE; Type 0: Not a Combination Product 2 NDC:11090-105-02 1 in 1 CARTON 03/14/2018 2 12 mL in 1 TUBE; Type 0: Not a Combination Product 3 NDC:11090-105-03 1 in 1 CARTON 03/14/2018 3 32 mL in 1 TUBE; Type 0: Not a Combination Product 4 NDC:11090-105-04 1 in 1 CARTON 03/14/2018 4 75 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part352 03/14/2018 ITC CC FULL COVERAGE CREAM MEDIUM
titanium dioxide, zinc oxide creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:11090-107 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 90 mg in 1 mL ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 63 mg in 1 mL Inactive Ingredients Ingredient Name Strength ALPHA-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) GLYCERIN (UNII: PDC6A3C0OX) 1,2-HEXANEDIOL (UNII: TR046Y3K1G) HEXYL LAURATE (UNII: 4CG9F9W01Q) STEARIC ACID (UNII: 4ELV7Z65AP) BOMBYX MORI FIBER (UNII: 6LK42KUV6W) DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER (SOFT PARTICLE) (UNII: 9E4CO0W6C5) PHENOXYETHANOL (UNII: HIE492ZZ3T) LEMON OIL (UNII: I9GRO824LL) STEARETH-20 (UNII: L0Q8IK9E08) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) FERRIC OXIDE RED (UNII: 1K09F3G675) PINE NEEDLE OIL (PINUS SYLVESTRIS) (UNII: 5EXL5H740Y) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) NIACINAMIDE (UNII: 25X51I8RD4) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) EUCALYPTUS OIL (UNII: 2R04ONI662) CHRYSIN (UNII: 3CN01F5ZJ5) BUTYLENE GLYCOL DICAPRYLATE/DICAPRATE (UNII: 75D21FL1PI) CALCIUM STEARATE (UNII: 776XM7047L) BABASSU OIL (UNII: 8QSB4M5477) DIMETHICONE (UNII: 92RU3N3Y1O) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) ADENOSINE (UNII: K72T3FS567) GREEN TEA LEAF (UNII: W2ZU1RY8B0) CYCLOMETHICONE 6 (UNII: XHK3U310BA) PHENYL TRIMETHICONE (UNII: DR0K5NOJ4R) MAGNESIUM SULFATE HEPTAHYDRATE (UNII: SK47B8698T) LIME OIL (UNII: UZH29XGA8G) FERROSOFERRIC OXIDE (UNII: XM0M87F357) CETYL PEG/PPG-10/1 DIMETHICONE (HLB 5) (UNII: 035JKJ76MT) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) POLYGLYCERYL-4 ISOSTEARATE (UNII: 820DPX33S7) OATMEAL (UNII: 8PI54V663Y) BERGAMOT OIL (UNII: 39W1PKE3JI) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) CAPRYLYL GLYCOL (UNII: 00YIU5438U) SORBITAN ISOSTEARATE (UNII: 01S2G2C1E4) WATER (UNII: 059QF0KO0R) GELATIN HYDROLYSATE (PORCINE SKIN, MW 3000) (UNII: 0K9R94573C) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) ORANGE OIL (UNII: AKN3KSD11B) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) OLEA EUROPAEA LEAF (UNII: MJ95C3OH47) N-HYDROXYSUCCINIMIDE (UNII: MJE3791M4T) CHLORHEXIDINE GLUCONATE (UNII: MOR84MUD8E) PALMITOYL TETRAPEPTIDE-7 (UNII: Q41S464P1R) PALMITOYL TRIPEPTIDE-1 (UNII: RV743D216M) HYALURONIC ACID (UNII: S270N0TRQY) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:11090-107-01 1 in 1 CARTON 03/14/2018 1 4 mL in 1 TUBE; Type 0: Not a Combination Product 2 NDC:11090-107-02 1 in 1 CARTON 03/14/2018 2 12 mL in 1 TUBE; Type 0: Not a Combination Product 3 NDC:11090-107-03 1 in 1 CARTON 03/14/2018 3 32 mL in 1 TUBE; Type 0: Not a Combination Product 4 NDC:11090-107-04 1 in 1 CARTON 03/14/2018 4 75 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part352 03/14/2018 ITC CC FULL COVERAGE CREAM MEDIUM TAN
titanium dioxide, zinc oxide creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:11090-109 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 90 mg in 1 mL ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 63 mg in 1 mL Inactive Ingredients Ingredient Name Strength ALPHA-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) GLYCERIN (UNII: PDC6A3C0OX) 1,2-HEXANEDIOL (UNII: TR046Y3K1G) HEXYL LAURATE (UNII: 4CG9F9W01Q) STEARIC ACID (UNII: 4ELV7Z65AP) BOMBYX MORI FIBER (UNII: 6LK42KUV6W) DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER (SOFT PARTICLE) (UNII: 9E4CO0W6C5) PHENOXYETHANOL (UNII: HIE492ZZ3T) LEMON OIL (UNII: I9GRO824LL) STEARETH-20 (UNII: L0Q8IK9E08) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) FERRIC OXIDE RED (UNII: 1K09F3G675) PINE NEEDLE OIL (PINUS SYLVESTRIS) (UNII: 5EXL5H740Y) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) NIACINAMIDE (UNII: 25X51I8RD4) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) EUCALYPTUS OIL (UNII: 2R04ONI662) CHRYSIN (UNII: 3CN01F5ZJ5) BUTYLENE GLYCOL DICAPRYLATE/DICAPRATE (UNII: 75D21FL1PI) CALCIUM STEARATE (UNII: 776XM7047L) BABASSU OIL (UNII: 8QSB4M5477) DIMETHICONE (UNII: 92RU3N3Y1O) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) ADENOSINE (UNII: K72T3FS567) GREEN TEA LEAF (UNII: W2ZU1RY8B0) CYCLOMETHICONE 6 (UNII: XHK3U310BA) PHENYL TRIMETHICONE (UNII: DR0K5NOJ4R) MAGNESIUM SULFATE HEPTAHYDRATE (UNII: SK47B8698T) LIME OIL (UNII: UZH29XGA8G) FERROSOFERRIC OXIDE (UNII: XM0M87F357) CETYL PEG/PPG-10/1 DIMETHICONE (HLB 5) (UNII: 035JKJ76MT) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) POLYGLYCERYL-4 ISOSTEARATE (UNII: 820DPX33S7) OATMEAL (UNII: 8PI54V663Y) BERGAMOT OIL (UNII: 39W1PKE3JI) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) CAPRYLYL GLYCOL (UNII: 00YIU5438U) SORBITAN ISOSTEARATE (UNII: 01S2G2C1E4) WATER (UNII: 059QF0KO0R) GELATIN HYDROLYSATE (PORCINE SKIN, MW 3000) (UNII: 0K9R94573C) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) ORANGE OIL (UNII: AKN3KSD11B) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) OLEA EUROPAEA LEAF (UNII: MJ95C3OH47) N-HYDROXYSUCCINIMIDE (UNII: MJE3791M4T) CHLORHEXIDINE GLUCONATE (UNII: MOR84MUD8E) PALMITOYL TETRAPEPTIDE-7 (UNII: Q41S464P1R) PALMITOYL TRIPEPTIDE-1 (UNII: RV743D216M) HYALURONIC ACID (UNII: S270N0TRQY) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:11090-109-01 1 in 1 CARTON 03/14/2018 1 4 mL in 1 TUBE; Type 0: Not a Combination Product 2 NDC:11090-109-02 1 in 1 CARTON 03/14/2018 2 12 mL in 1 TUBE; Type 0: Not a Combination Product 3 NDC:11090-109-03 1 in 1 CARTON 03/14/2018 3 32 mL in 1 TUBE; Type 0: Not a Combination Product 4 NDC:11090-109-04 1 in 1 CARTON 03/14/2018 4 75 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part352 03/14/2018 ITC CC FULL COVERAGE CREAM TAN
titanium dioxide, zinc oxide creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:11090-111 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 90 mg in 1 mL ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 63 mg in 1 mL Inactive Ingredients Ingredient Name Strength ALPHA-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) GLYCERIN (UNII: PDC6A3C0OX) 1,2-HEXANEDIOL (UNII: TR046Y3K1G) HEXYL LAURATE (UNII: 4CG9F9W01Q) STEARIC ACID (UNII: 4ELV7Z65AP) BOMBYX MORI FIBER (UNII: 6LK42KUV6W) DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER (SOFT PARTICLE) (UNII: 9E4CO0W6C5) PHENOXYETHANOL (UNII: HIE492ZZ3T) LEMON OIL (UNII: I9GRO824LL) STEARETH-20 (UNII: L0Q8IK9E08) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) FERRIC OXIDE RED (UNII: 1K09F3G675) PINE NEEDLE OIL (PINUS SYLVESTRIS) (UNII: 5EXL5H740Y) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) NIACINAMIDE (UNII: 25X51I8RD4) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) EUCALYPTUS OIL (UNII: 2R04ONI662) CHRYSIN (UNII: 3CN01F5ZJ5) BUTYLENE GLYCOL DICAPRYLATE/DICAPRATE (UNII: 75D21FL1PI) CALCIUM STEARATE (UNII: 776XM7047L) BABASSU OIL (UNII: 8QSB4M5477) DIMETHICONE (UNII: 92RU3N3Y1O) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) ADENOSINE (UNII: K72T3FS567) GREEN TEA LEAF (UNII: W2ZU1RY8B0) CYCLOMETHICONE 6 (UNII: XHK3U310BA) PHENYL TRIMETHICONE (UNII: DR0K5NOJ4R) MAGNESIUM SULFATE HEPTAHYDRATE (UNII: SK47B8698T) LIME OIL (UNII: UZH29XGA8G) FERROSOFERRIC OXIDE (UNII: XM0M87F357) CETYL PEG/PPG-10/1 DIMETHICONE (HLB 5) (UNII: 035JKJ76MT) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) POLYGLYCERYL-4 ISOSTEARATE (UNII: 820DPX33S7) OATMEAL (UNII: 8PI54V663Y) BERGAMOT OIL (UNII: 39W1PKE3JI) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) CAPRYLYL GLYCOL (UNII: 00YIU5438U) SORBITAN ISOSTEARATE (UNII: 01S2G2C1E4) WATER (UNII: 059QF0KO0R) GELATIN HYDROLYSATE (PORCINE SKIN, MW 3000) (UNII: 0K9R94573C) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) ORANGE OIL (UNII: AKN3KSD11B) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) OLEA EUROPAEA LEAF (UNII: MJ95C3OH47) N-HYDROXYSUCCINIMIDE (UNII: MJE3791M4T) CHLORHEXIDINE GLUCONATE (UNII: MOR84MUD8E) PALMITOYL TETRAPEPTIDE-7 (UNII: Q41S464P1R) PALMITOYL TRIPEPTIDE-1 (UNII: RV743D216M) HYALURONIC ACID (UNII: S270N0TRQY) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:11090-111-01 1 in 1 CARTON 03/14/2018 1 4 mL in 1 TUBE; Type 0: Not a Combination Product 2 NDC:11090-111-02 1 in 1 CARTON 03/14/2018 2 12 mL in 1 TUBE; Type 0: Not a Combination Product 3 NDC:11090-111-03 1 in 1 CARTON 03/14/2018 3 32 mL in 1 TUBE; Type 0: Not a Combination Product 4 NDC:11090-111-04 1 in 1 CARTON 03/14/2018 4 75 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part352 03/14/2018 ITC CC FULL COVERAGE CREAM RICH HONEY
titanium dioxide, zinc oxide creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:11090-113 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 90 mg in 1 mL ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 63 mg in 1 mL Inactive Ingredients Ingredient Name Strength ALPHA-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) GLYCERIN (UNII: PDC6A3C0OX) 1,2-HEXANEDIOL (UNII: TR046Y3K1G) HEXYL LAURATE (UNII: 4CG9F9W01Q) STEARIC ACID (UNII: 4ELV7Z65AP) BOMBYX MORI FIBER (UNII: 6LK42KUV6W) DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER (SOFT PARTICLE) (UNII: 9E4CO0W6C5) PHENOXYETHANOL (UNII: HIE492ZZ3T) LEMON OIL (UNII: I9GRO824LL) STEARETH-20 (UNII: L0Q8IK9E08) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) FERRIC OXIDE RED (UNII: 1K09F3G675) PINE NEEDLE OIL (PINUS SYLVESTRIS) (UNII: 5EXL5H740Y) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) NIACINAMIDE (UNII: 25X51I8RD4) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) EUCALYPTUS OIL (UNII: 2R04ONI662) CHRYSIN (UNII: 3CN01F5ZJ5) BUTYLENE GLYCOL DICAPRYLATE/DICAPRATE (UNII: 75D21FL1PI) CALCIUM STEARATE (UNII: 776XM7047L) BABASSU OIL (UNII: 8QSB4M5477) DIMETHICONE (UNII: 92RU3N3Y1O) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) ADENOSINE (UNII: K72T3FS567) GREEN TEA LEAF (UNII: W2ZU1RY8B0) CYCLOMETHICONE 6 (UNII: XHK3U310BA) PHENYL TRIMETHICONE (UNII: DR0K5NOJ4R) MAGNESIUM SULFATE HEPTAHYDRATE (UNII: SK47B8698T) LIME OIL (UNII: UZH29XGA8G) FERROSOFERRIC OXIDE (UNII: XM0M87F357) CETYL PEG/PPG-10/1 DIMETHICONE (HLB 5) (UNII: 035JKJ76MT) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) POLYGLYCERYL-4 ISOSTEARATE (UNII: 820DPX33S7) OATMEAL (UNII: 8PI54V663Y) BERGAMOT OIL (UNII: 39W1PKE3JI) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) CAPRYLYL GLYCOL (UNII: 00YIU5438U) SORBITAN ISOSTEARATE (UNII: 01S2G2C1E4) WATER (UNII: 059QF0KO0R) GELATIN HYDROLYSATE (PORCINE SKIN, MW 3000) (UNII: 0K9R94573C) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) ORANGE OIL (UNII: AKN3KSD11B) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) OLEA EUROPAEA LEAF (UNII: MJ95C3OH47) N-HYDROXYSUCCINIMIDE (UNII: MJE3791M4T) CHLORHEXIDINE GLUCONATE (UNII: MOR84MUD8E) PALMITOYL TETRAPEPTIDE-7 (UNII: Q41S464P1R) PALMITOYL TRIPEPTIDE-1 (UNII: RV743D216M) HYALURONIC ACID (UNII: S270N0TRQY) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:11090-113-01 1 in 1 CARTON 03/14/2018 1 12 mL in 1 TUBE; Type 0: Not a Combination Product 2 NDC:11090-113-03 1 in 1 CARTON 03/14/2018 2 75 mL in 1 TUBE; Type 0: Not a Combination Product 3 NDC:11090-113-02 1 in 1 CARTON 03/14/2018 3 32 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part352 03/14/2018 ITC CC FULL COVERAGE CREAM FAIR
titanium dioxide, zinc oxide creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:11090-103 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 90 mg in 1 mL ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 63 mg in 1 mL Inactive Ingredients Ingredient Name Strength ALPHA-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) GLYCERIN (UNII: PDC6A3C0OX) 1,2-HEXANEDIOL (UNII: TR046Y3K1G) HEXYL LAURATE (UNII: 4CG9F9W01Q) STEARIC ACID (UNII: 4ELV7Z65AP) BOMBYX MORI FIBER (UNII: 6LK42KUV6W) DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER (SOFT PARTICLE) (UNII: 9E4CO0W6C5) PHENOXYETHANOL (UNII: HIE492ZZ3T) LEMON OIL (UNII: I9GRO824LL) STEARETH-20 (UNII: L0Q8IK9E08) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) FERRIC OXIDE RED (UNII: 1K09F3G675) PINE NEEDLE OIL (PINUS SYLVESTRIS) (UNII: 5EXL5H740Y) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) NIACINAMIDE (UNII: 25X51I8RD4) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) EUCALYPTUS OIL (UNII: 2R04ONI662) CHRYSIN (UNII: 3CN01F5ZJ5) BUTYLENE GLYCOL DICAPRYLATE/DICAPRATE (UNII: 75D21FL1PI) CALCIUM STEARATE (UNII: 776XM7047L) BABASSU OIL (UNII: 8QSB4M5477) DIMETHICONE (UNII: 92RU3N3Y1O) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) ADENOSINE (UNII: K72T3FS567) GREEN TEA LEAF (UNII: W2ZU1RY8B0) CYCLOMETHICONE 6 (UNII: XHK3U310BA) PHENYL TRIMETHICONE (UNII: DR0K5NOJ4R) MAGNESIUM SULFATE HEPTAHYDRATE (UNII: SK47B8698T) LIME OIL (UNII: UZH29XGA8G) FERROSOFERRIC OXIDE (UNII: XM0M87F357) CETYL PEG/PPG-10/1 DIMETHICONE (HLB 5) (UNII: 035JKJ76MT) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) POLYGLYCERYL-4 ISOSTEARATE (UNII: 820DPX33S7) OATMEAL (UNII: 8PI54V663Y) BERGAMOT OIL (UNII: 39W1PKE3JI) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) CAPRYLYL GLYCOL (UNII: 00YIU5438U) SORBITAN ISOSTEARATE (UNII: 01S2G2C1E4) WATER (UNII: 059QF0KO0R) GELATIN HYDROLYSATE (PORCINE SKIN, MW 3000) (UNII: 0K9R94573C) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) ORANGE OIL (UNII: AKN3KSD11B) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) OLEA EUROPAEA LEAF (UNII: MJ95C3OH47) N-HYDROXYSUCCINIMIDE (UNII: MJE3791M4T) CHLORHEXIDINE GLUCONATE (UNII: MOR84MUD8E) PALMITOYL TETRAPEPTIDE-7 (UNII: Q41S464P1R) PALMITOYL TRIPEPTIDE-1 (UNII: RV743D216M) HYALURONIC ACID (UNII: S270N0TRQY) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:11090-103-01 1 in 1 CARTON 03/14/2018 1 4 mL in 1 TUBE; Type 0: Not a Combination Product 2 NDC:11090-103-02 1 in 1 CARTON 03/14/2018 2 12 mL in 1 TUBE; Type 0: Not a Combination Product 3 NDC:11090-103-03 1 in 1 CARTON 03/14/2018 3 32 mL in 1 TUBE; Type 0: Not a Combination Product 4 NDC:11090-103-04 1 in 1 CARTON 03/14/2018 4 75 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part352 03/14/2018 ITC CC FULL COVERAGE CREAM LIGHT MEDIUM
titanium dioxide, zinc oxide creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:11090-106 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 90 mg in 1 mL ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 63 mg in 1 mL Inactive Ingredients Ingredient Name Strength ALPHA-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) GLYCERIN (UNII: PDC6A3C0OX) 1,2-HEXANEDIOL (UNII: TR046Y3K1G) HEXYL LAURATE (UNII: 4CG9F9W01Q) STEARIC ACID (UNII: 4ELV7Z65AP) BOMBYX MORI FIBER (UNII: 6LK42KUV6W) DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER (SOFT PARTICLE) (UNII: 9E4CO0W6C5) PHENOXYETHANOL (UNII: HIE492ZZ3T) LEMON OIL (UNII: I9GRO824LL) STEARETH-20 (UNII: L0Q8IK9E08) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) FERRIC OXIDE RED (UNII: 1K09F3G675) PINE NEEDLE OIL (PINUS SYLVESTRIS) (UNII: 5EXL5H740Y) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) NIACINAMIDE (UNII: 25X51I8RD4) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) EUCALYPTUS OIL (UNII: 2R04ONI662) CHRYSIN (UNII: 3CN01F5ZJ5) BUTYLENE GLYCOL DICAPRYLATE/DICAPRATE (UNII: 75D21FL1PI) CALCIUM STEARATE (UNII: 776XM7047L) BABASSU OIL (UNII: 8QSB4M5477) DIMETHICONE (UNII: 92RU3N3Y1O) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) ADENOSINE (UNII: K72T3FS567) GREEN TEA LEAF (UNII: W2ZU1RY8B0) CYCLOMETHICONE 6 (UNII: XHK3U310BA) PHENYL TRIMETHICONE (UNII: DR0K5NOJ4R) MAGNESIUM SULFATE HEPTAHYDRATE (UNII: SK47B8698T) LIME OIL (UNII: UZH29XGA8G) FERROSOFERRIC OXIDE (UNII: XM0M87F357) CETYL PEG/PPG-10/1 DIMETHICONE (HLB 5) (UNII: 035JKJ76MT) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) POLYGLYCERYL-4 ISOSTEARATE (UNII: 820DPX33S7) OATMEAL (UNII: 8PI54V663Y) BERGAMOT OIL (UNII: 39W1PKE3JI) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) CAPRYLYL GLYCOL (UNII: 00YIU5438U) SORBITAN ISOSTEARATE (UNII: 01S2G2C1E4) WATER (UNII: 059QF0KO0R) GELATIN HYDROLYSATE (PORCINE SKIN, MW 3000) (UNII: 0K9R94573C) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) ORANGE OIL (UNII: AKN3KSD11B) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) OLEA EUROPAEA LEAF (UNII: MJ95C3OH47) N-HYDROXYSUCCINIMIDE (UNII: MJE3791M4T) CHLORHEXIDINE GLUCONATE (UNII: MOR84MUD8E) PALMITOYL TETRAPEPTIDE-7 (UNII: Q41S464P1R) PALMITOYL TRIPEPTIDE-1 (UNII: RV743D216M) HYALURONIC ACID (UNII: S270N0TRQY) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:11090-106-01 1 in 1 CARTON 03/14/2018 1 12 mL in 1 TUBE; Type 0: Not a Combination Product 2 NDC:11090-106-03 1 in 1 CARTON 03/14/2018 2 75 mL in 1 TUBE; Type 0: Not a Combination Product 3 NDC:11090-106-02 1 in 1 CARTON 03/14/2018 3 32 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part352 03/14/2018 ITC CC FULL COVERAGE CREAM RICH
titanium dioxide, zinc oxide creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:11090-112 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 90 mg in 1 mL ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 63 mg in 1 mL Inactive Ingredients Ingredient Name Strength ALPHA-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) GLYCERIN (UNII: PDC6A3C0OX) 1,2-HEXANEDIOL (UNII: TR046Y3K1G) HEXYL LAURATE (UNII: 4CG9F9W01Q) STEARIC ACID (UNII: 4ELV7Z65AP) BOMBYX MORI FIBER (UNII: 6LK42KUV6W) DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER (SOFT PARTICLE) (UNII: 9E4CO0W6C5) PHENOXYETHANOL (UNII: HIE492ZZ3T) LEMON OIL (UNII: I9GRO824LL) STEARETH-20 (UNII: L0Q8IK9E08) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) FERRIC OXIDE RED (UNII: 1K09F3G675) PINE NEEDLE OIL (PINUS SYLVESTRIS) (UNII: 5EXL5H740Y) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) NIACINAMIDE (UNII: 25X51I8RD4) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) EUCALYPTUS OIL (UNII: 2R04ONI662) CHRYSIN (UNII: 3CN01F5ZJ5) BUTYLENE GLYCOL DICAPRYLATE/DICAPRATE (UNII: 75D21FL1PI) CALCIUM STEARATE (UNII: 776XM7047L) BABASSU OIL (UNII: 8QSB4M5477) DIMETHICONE (UNII: 92RU3N3Y1O) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) ADENOSINE (UNII: K72T3FS567) GREEN TEA LEAF (UNII: W2ZU1RY8B0) CYCLOMETHICONE 6 (UNII: XHK3U310BA) PHENYL TRIMETHICONE (UNII: DR0K5NOJ4R) MAGNESIUM SULFATE HEPTAHYDRATE (UNII: SK47B8698T) LIME OIL (UNII: UZH29XGA8G) FERROSOFERRIC OXIDE (UNII: XM0M87F357) CETYL PEG/PPG-10/1 DIMETHICONE (HLB 5) (UNII: 035JKJ76MT) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) POLYGLYCERYL-4 ISOSTEARATE (UNII: 820DPX33S7) OATMEAL (UNII: 8PI54V663Y) BERGAMOT OIL (UNII: 39W1PKE3JI) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) CAPRYLYL GLYCOL (UNII: 00YIU5438U) SORBITAN ISOSTEARATE (UNII: 01S2G2C1E4) WATER (UNII: 059QF0KO0R) GELATIN HYDROLYSATE (PORCINE SKIN, MW 3000) (UNII: 0K9R94573C) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) ORANGE OIL (UNII: AKN3KSD11B) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) OLEA EUROPAEA LEAF (UNII: MJ95C3OH47) N-HYDROXYSUCCINIMIDE (UNII: MJE3791M4T) CHLORHEXIDINE GLUCONATE (UNII: MOR84MUD8E) PALMITOYL TETRAPEPTIDE-7 (UNII: Q41S464P1R) PALMITOYL TRIPEPTIDE-1 (UNII: RV743D216M) HYALURONIC ACID (UNII: S270N0TRQY) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:11090-112-01 1 in 1 CARTON 03/14/2018 1 4 mL in 1 TUBE; Type 0: Not a Combination Product 2 NDC:11090-112-02 1 in 1 CARTON 03/14/2018 2 12 mL in 1 TUBE; Type 0: Not a Combination Product 3 NDC:11090-112-03 1 in 1 CARTON 03/14/2018 3 32 mL in 1 TUBE; Type 0: Not a Combination Product 4 NDC:11090-112-04 1 in 1 CARTON 03/14/2018 4 75 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part352 03/14/2018 Labeler - Beauty Manufacturing Solutions Corp. (783200723) Registrant - Beauty Manufacturing Solutions Corp. (783200723) Establishment Name Address ID/FEI Business Operations Beauty Manufacturing Solutions Corp. 783200723 manufacture(11090-103, 11090-104, 11090-105, 11090-106, 11090-107, 11090-108, 11090-109, 11090-110, 11090-111, 11090-112, 11090-113)