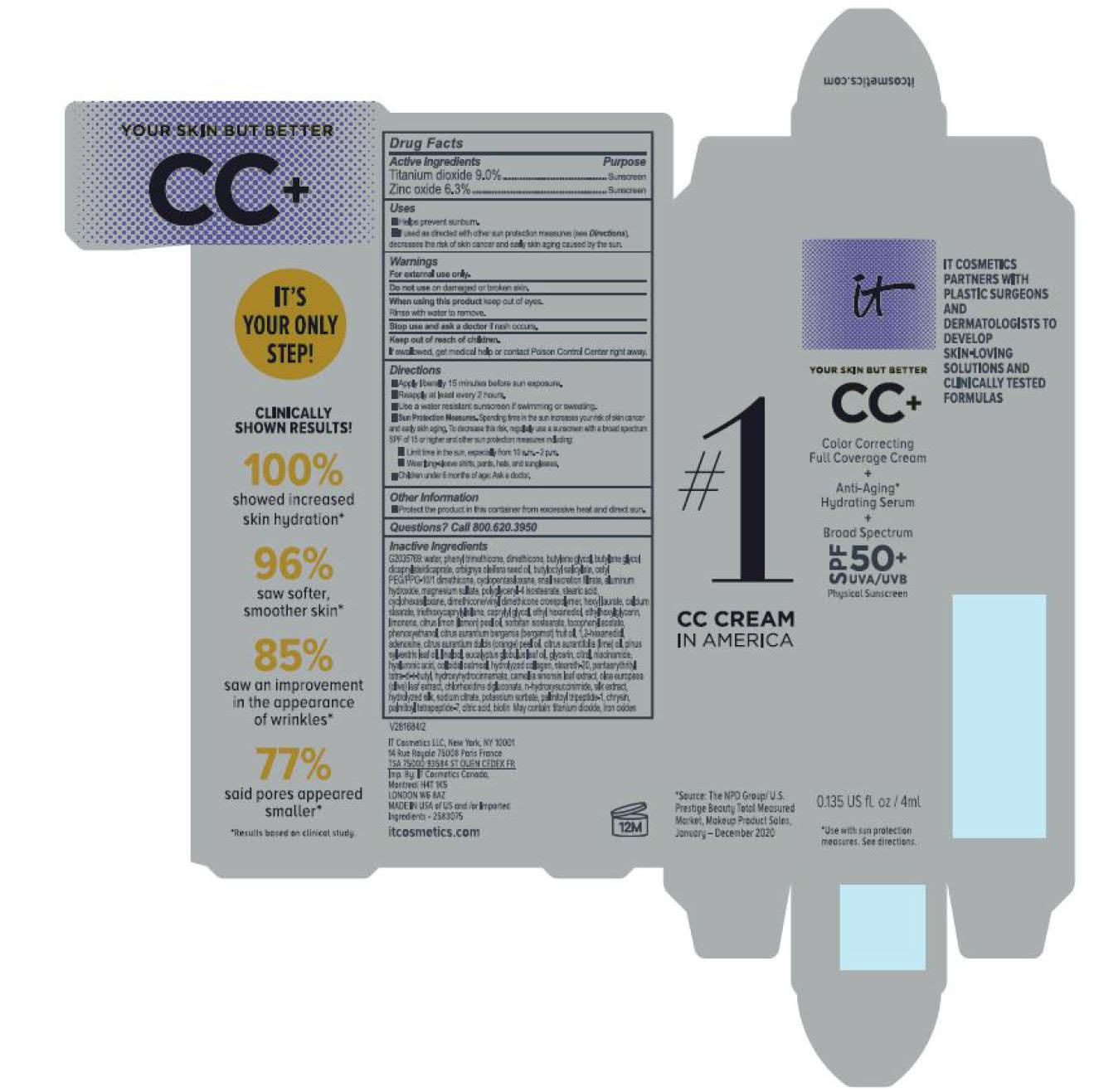
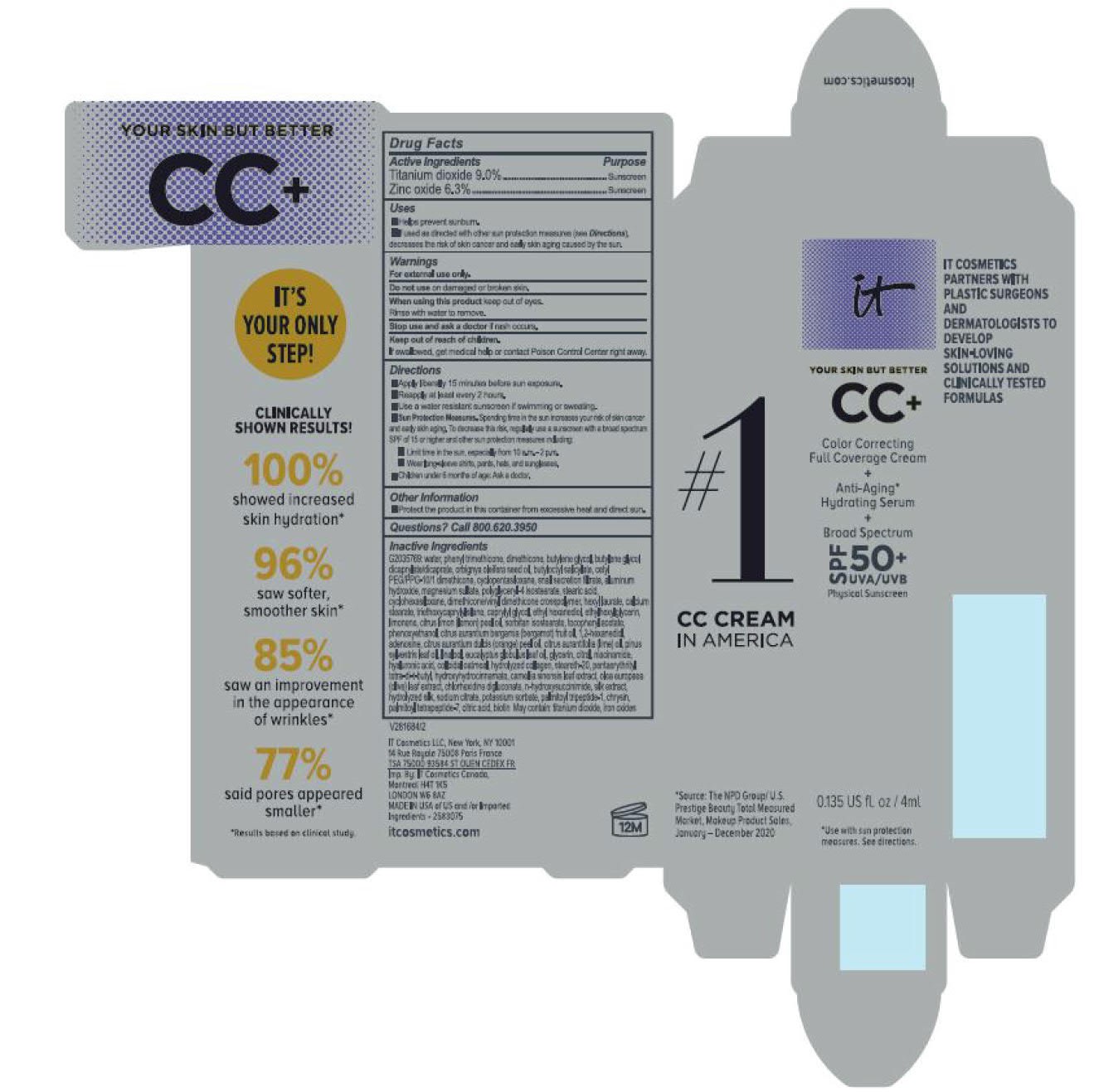
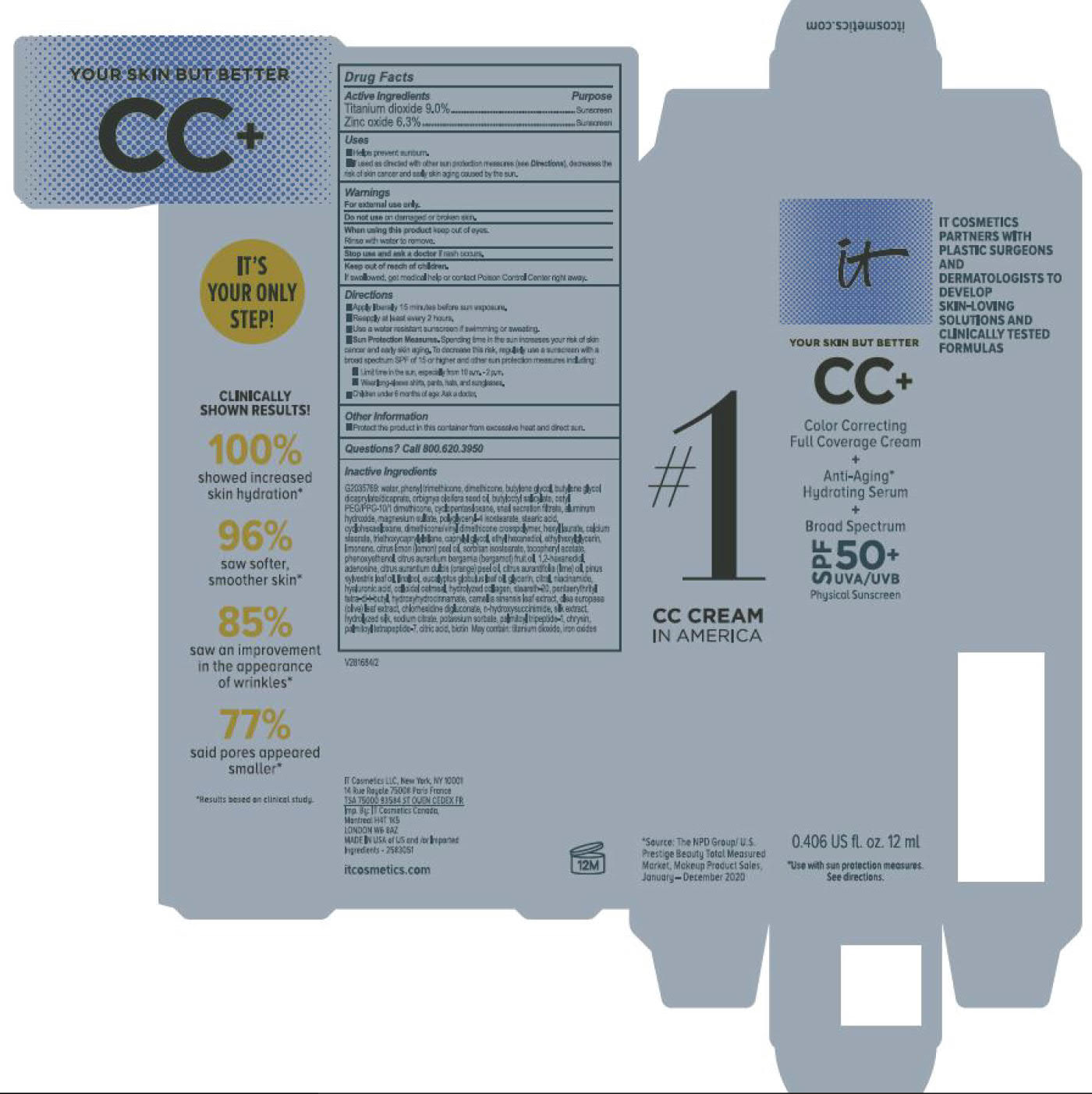
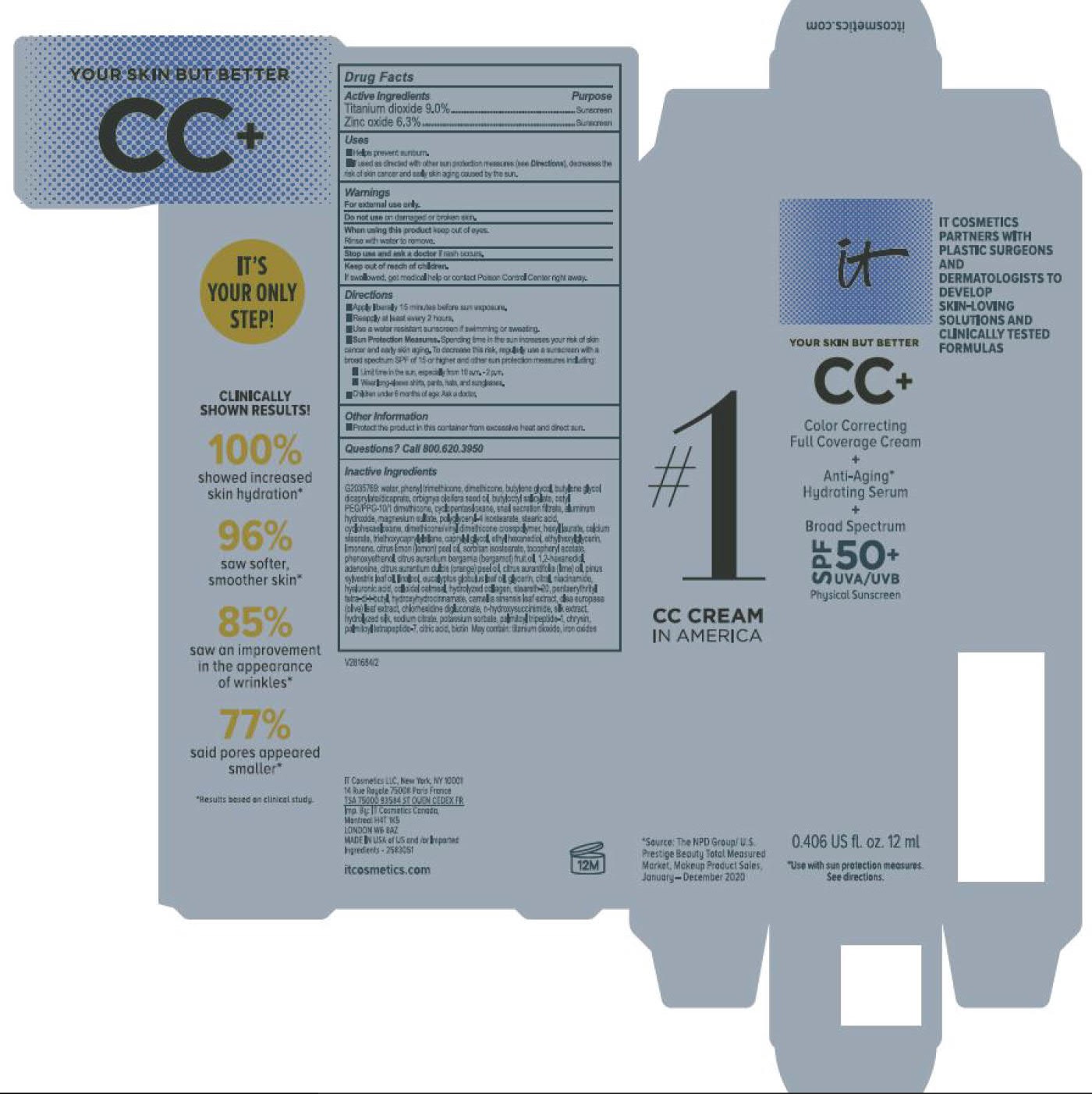
- helps prevent sunburn
- if used as directed with other sun protection measures (see Directions), decreases the risk of skin cancer and early skin aging caused by the sun
For sunscreen use:
● apply liberally 15 minutes before sun exposure
● reapply at least every 2 hours
● use a water resistant sunscreen if swimming or sweating
● Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including:
● limit time in the sun, especially from 10 a.m. – 2 p.m.
● wear long-sleeved shirts, pants, hats, and sunglasses
● children under 6 months of age: Ask a doctor
water, snail secretion filtrate, phenyl trimethicone, dimethicone, butylene glycol, butylene glycol dicaprylated/dicaprate, orbignya oleifera seed oil, butyloctyl salicylate, cetyl PEG/PPG-10/1 dimethiocone, cyclopentasiloxane, cyclohexasiloxane, magnesium sulfate, polyglyceryl-4 isostearate, dimethicone/vinyl dimethicone crosspolymer, aluminum hydroxide, hexyl laurate, stearic acid, calcium stearate, caprylyl glycol, triethoxycaprylylsilane, ethylhexylglycerin, citrus medica limonum (lemon) peel oil, tocopheryl acetate, sorbitan isostearate, phenoxyethanol, citrus aurantium bergamia (bergamot) fruit oil, 1,2-hexanediol, disodium EDTA, citrus aurantium dulcis (orange) peel oil, cirus aurantifolia (lime) oil vitis vinifera (grape) seed oil, punica gratissiima (avocado) oil, niacinamide, citrus grandis (grapefuit) peel oil, chloesterol, anthemis b=nobilis flower water, lactobacillius/honeysuckle flowr/locorice root/morus alba root/pueraria lobata root/schizandra chinensis fruit/scutellaria baicalensis root/sophora japonica flower extract ferment filtrate, perfluorohexane, olea europaea (olive) leaf extract, glycerin, eucalyptus globulus leaf oil, camellia sinensis leaf extract, chrysanthemium indicum flower extract, pueraria lobata root extract, perfluorodecalin, morus alba gruit extract, magnolia kobus bark extract, glycine soja (soybean) sprout extract, diospyros kaki leaf extract, cinnamomum cassia bark extract, artemisia princeps leaf extract, epntafluoropropane, curcuma longa (turmeric) root extract, steareth-20, hydrolyzed silk, hydrolyzed hyaluronic acid, colloidal oatmeal, citric acid, sodium benzoate, potassum sorbate, aloe barbadensis leaf extract, n-hydroxysuccinimide, hydrolyzed collagen, caprylhydroxamic acid, tocopherol, thiamine HCL, riboflavin, retinyl palmitate, pantothenic acid, palmitoyl oligopeptide, nicin, folic acid, chrysin, carnitine HCL, biotin, ascorbic acid, palmitoyl tetrapeptide-7, chlorhexidine digluconate; may contain: iron oxides