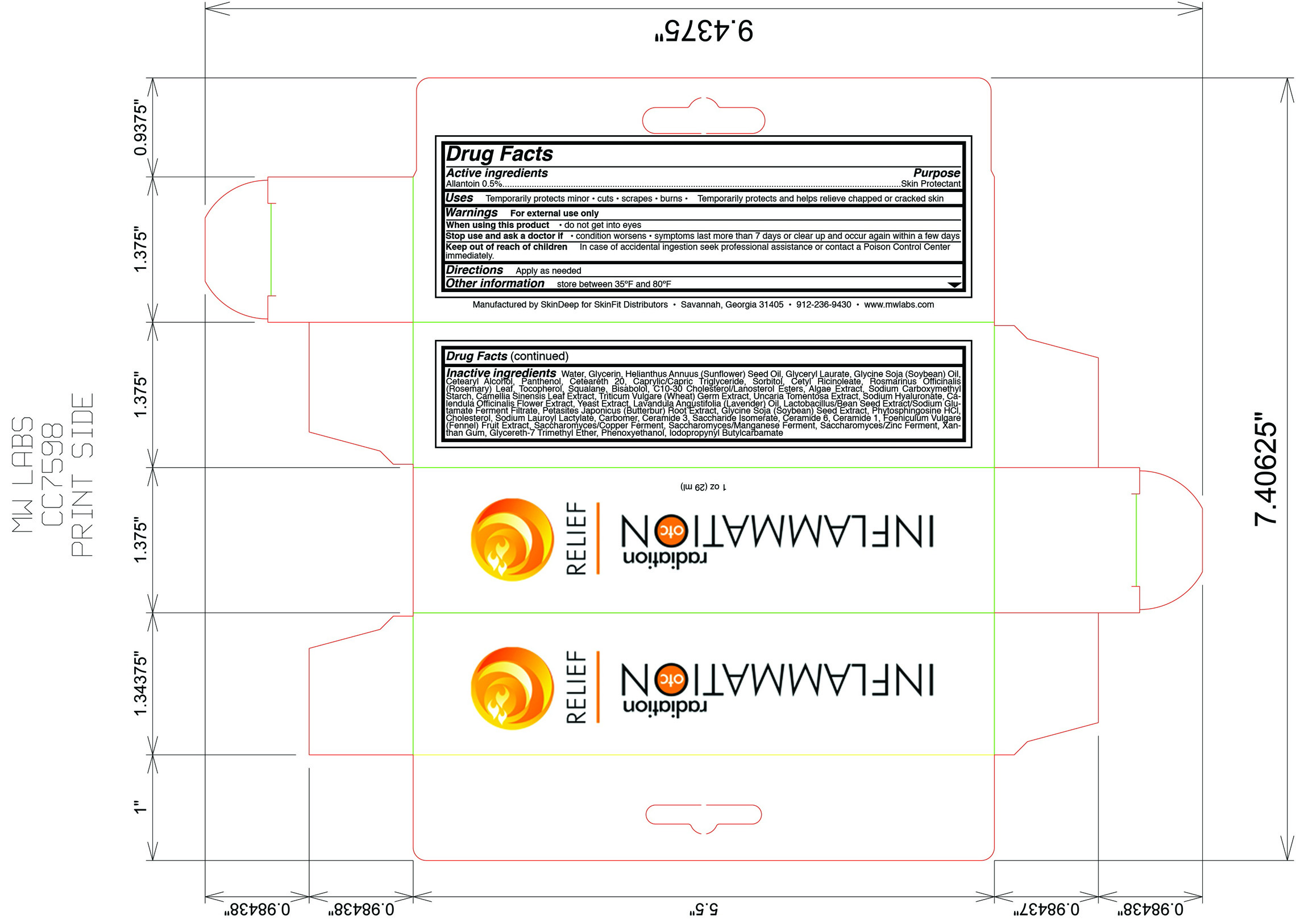
Label: INFLAMMATION OTC- allantoin lotion
-
Contains inactivated NDC Code(s)
NDC Code(s): 45156-5616-1, 45156-5616-2 - Packager: Skin Deep
- Category: HUMAN OTC DRUG LABEL
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated January 16, 2011
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient
- Purpose
- Uses
- Warnings
- Keep out of reach of children
- Directions
-
Inactive ingredients
water, glycerin, helianthus annuus (sunflower) seed oil, glyceryl laurate, glycine soja (soybean) oil, cetearyl alcohol, panthenol, ceteareth 20, caprylic/capric triglyeride, sorbitol, cetyl ricinoleate, rosmarinus officinalis (rosemary) leaf, tocopherol, squalane, bisabolol, C10-30 cholesterol/lanosterol esters, algae extract,sodium carboxymethly starch, camelia sinensis leaf extract, triticum vulgare (wheat) germ extract, uncaria tomentosa extract, sodium hyaluronate, calendula officinalis flower extract, yeast extract, lavandula angustifolia (lavender) oil, lactobacillus/bean seed extract/sodium glutamate ferment filtrate, petasites japonicus (butterbur) root extract, glycine soja (soybean) seed extract, phytosphingosine HCl, cholesterol, sodium lauroyl lactylate, carbomer, ceramide 3, saccharide isomerate, ceramide 6, ceramide 1, foeniculum vulgare (fennel) fruit extract, saccharomyces/copper ferment, saccharomyces/manganese ferment, saccharomyces/zinc ferment, xanthan gum, glycereth-7 trimethyl ether, phenoxyethanol, iodopropynyl butylcarbmate
- Questions or comments
- Principal Display Panel
-
INGREDIENTS AND APPEARANCE
INFLAMMATION OTC RADIATION RELIEF
allantoin lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:45156-5616 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Allantoin (UNII: 344S277G0Z) (Allantoin - UNII:344S277G0Z) Allantoin 3 g in 600 g Inactive Ingredients Ingredient Name Strength Water (UNII: 059QF0KO0R) Glycerin (UNII: PDC6A3C0OX) Helianthus Annuus (UNII: BKJ0J3D1BP) Glyceryl Laurate (UNII: Y98611C087) Soybean Oil (UNII: 241ATL177A) Cetostearyl Alcohol (UNII: 2DMT128M1S) Dexpanthenol (UNII: 1O6C93RI7Z) Polyoxyl 20 Cetostearyl Ether (UNII: YRC528SWUY) Medium-Chain Triglycerides (UNII: C9H2L21V7U) Sorbitol (UNII: 506T60A25R) Ricinoleic Acid (UNII: I2D0F69854) Rosemary Oil (UNII: 8LGU7VM393) Alpha-Tocopherol (UNII: H4N855PNZ1) Squalane (UNII: GW89575KF9) .Alpha.-Bisabolol, (+/-)- (UNII: 36HQN158VC) Carrageenan (UNII: 5C69YCD2YJ) Sodium Starch Glycolate Type A Potato (UNII: 5856J3G2A2) Green Tea Leaf (UNII: W2ZU1RY8B0) Triticum Aestivum Pollen (UNII: F1KAH8374D) Cat's Claw (UNII: 9060PRM18Q) Hyaluronate Sodium (UNII: YSE9PPT4TH) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:45156-5616-2 1 in 1 BOX 1 NDC:45156-5616-1 29 g in 1 TUBE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part347 06/13/2010 Labeler - Skin Deep (833276566) Establishment Name Address ID/FEI Business Operations Skin Deep 833276566 manufacture