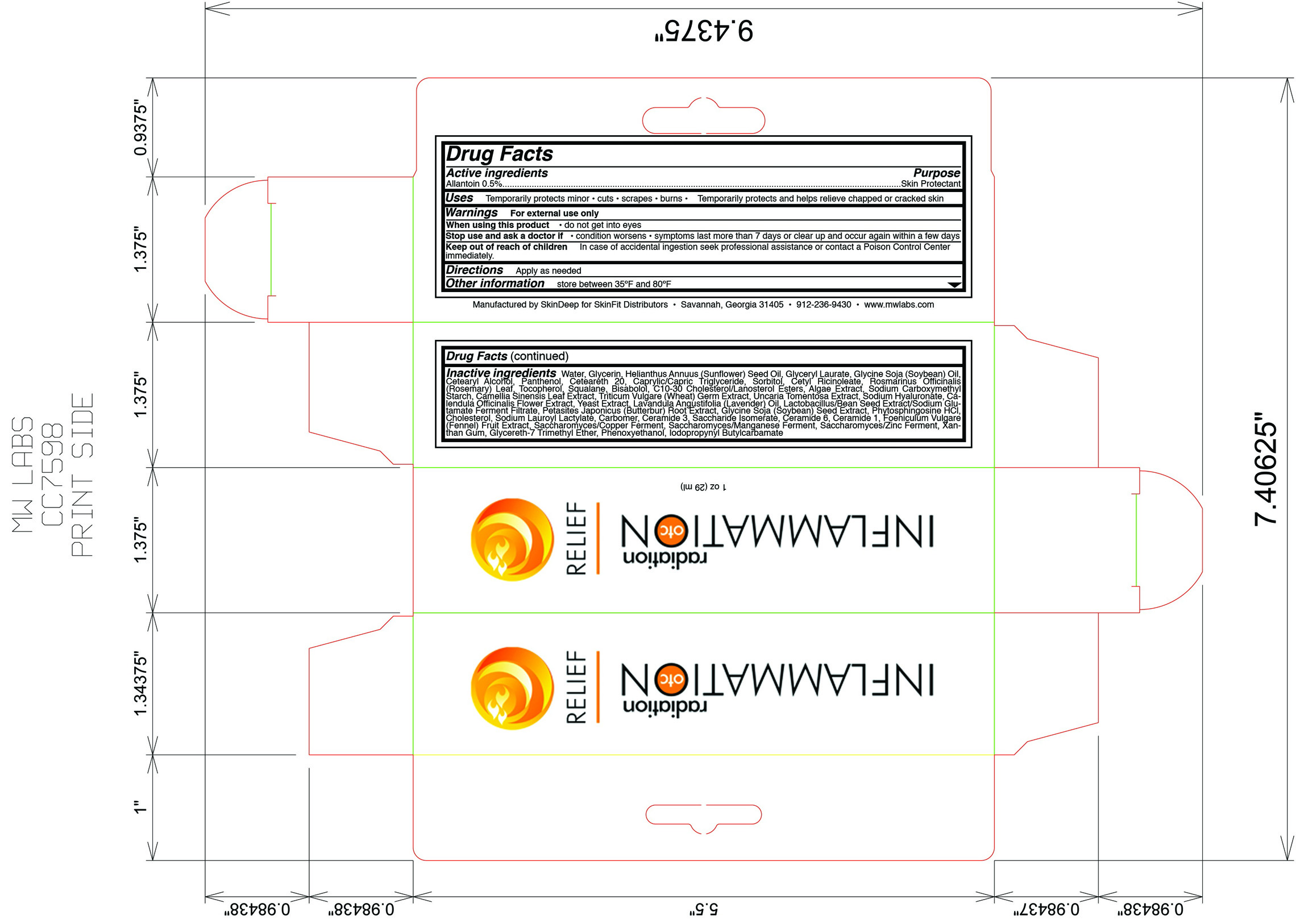
Uses
Temporarily protects minor: burns, cuts, and scrapes
- chapped skin
- cracked skin
Keep out of reach of children
In case of accidental ingestion seek professional assistance or contact a poison control center immediately.
Directions
- Apply as needed
- condition worsens
- symptoms last more than 7 days or clear up and occur again within a few days
Inactive ingredients
water, glycerin, helianthus annuus (sunflower) seed oil, glyceryl laurate, glycine soja (soybean) oil, cetearyl alcohol, panthenol, ceteareth 20, caprylic/capric triglyeride, sorbitol, cetyl ricinoleate, rosmarinus officinalis (rosemary) leaf, tocopherol, squalane, bisabolol, C10-30 cholesterol/lanosterol esters, algae extract,sodium carboxymethly starch, camelia sinensis leaf extract, triticum vulgare (wheat) germ extract, uncaria tomentosa extract, sodium hyaluronate, calendula officinalis flower extract, yeast extract, lavandula angustifolia (lavender) oil, lactobacillus/bean seed extract/sodium glutamate ferment filtrate, petasites japonicus (butterbur) root extract, glycine soja (soybean) seed extract, phytosphingosine HCl, cholesterol, sodium lauroyl lactylate, carbomer, ceramide 3, saccharide isomerate, ceramide 6, ceramide 1, foeniculum vulgare (fennel) fruit extract, saccharomyces/copper ferment, saccharomyces/manganese ferment, saccharomyces/zinc ferment, xanthan gum, glycereth-7 trimethyl ether, phenoxyethanol, iodopropynyl butylcarbmate