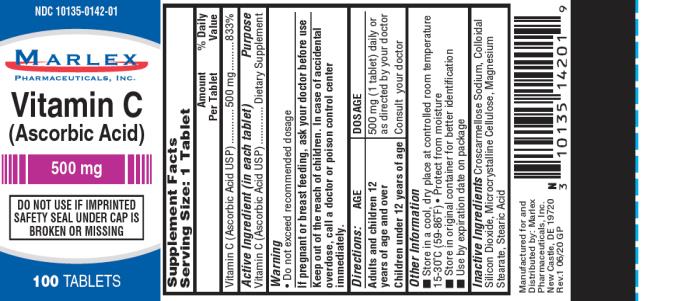
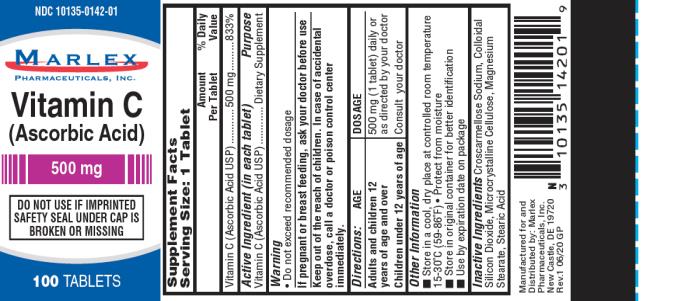
Label: VITAMIN C- ascorbic acid tablet
- NHRIC Code(s): 10135-142-01
- Packager: Marlex Pharmaceuticals Inc
- Category: DIETARY SUPPLEMENT
- DEA Schedule: None
- Marketing Status: Dietary Supplement
Drug Label Information
Updated July 1, 2020
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Supplement Facts
- Warning:
- Directions
-
Other Information:
- Store in a cool, dry place at controlled room temperature 15-30ºC (59-86ºF)
- Protect from moisture
- Store in original container for better identification
- Use by expiration date on package
Inactive Ingredients:
Microcrystalline Cellulose, Stearic Acid, Colloidal Silicon Dioxide, Magnesium Stearate, Croscarmellose Sodium.
Manufactured for/Distributed by:
Marlex Pharmaceuticals, Inc.,
New Castle, DE 19720Rev.1 6/20 GP
- Store in a cool, dry place at controlled room temperature 15-30ºC (59-86ºF)
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
VITAMIN C
ascorbic acid tabletProduct Information Product Type DIETARY SUPPLEMENT Item Code (Source) NHRIC:10135-142 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ASCORBIC ACID (UNII: PQ6CK8PD0R) (ASCORBIC ACID - UNII:PQ6CK8PD0R) ASCORBIC ACID 500 mg Inactive Ingredients Ingredient Name Strength MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) STEARIC ACID (UNII: 4ELV7Z65AP) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) MAGNESIUM STEARATE (UNII: 70097M6I30) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NHRIC:10135-142-01 100 in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date DIETARY SUPPLEMENT 06/01/2020 Supplement Facts Serving Size : Serving per Container : Amount Per Serving % Daily Value color imprint scoring 1 shape size (solid drugs) 12 mm Labeler - Marlex Pharmaceuticals Inc (782540215)