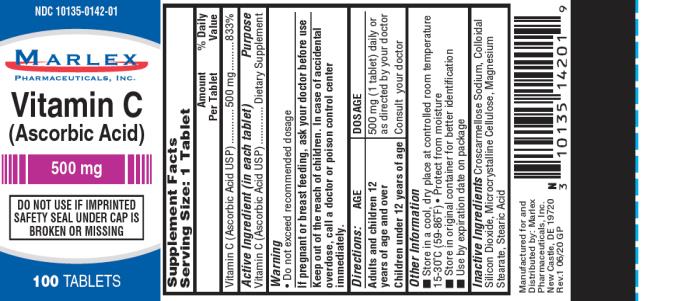
Supplement Facts
| Serving Size: 1 Tablet | ||
| Amount per Tablet | % Daily Value | |
| Vitamin C (Ascorbic Acid USP) | 500 mg | 833% |
| Active Ingredient (in each tablet) | Purpose | |
| Vitamin C (Ascorbic Acid USP) | Dietary Supplement |
Warning:
- Do not exceed recommended dosage.
If pregnant or breast-feeding,
ask your doctor before use.
Keep out of the reach of children.
In case of accidental overdose, call a doctor or poison control center immediately.
Directions
Adults and Children 12 years and over:
500 mg (1 tablet) daily or as directed by your doctor
Children under 12 years of age:
Consult your doctor
Other Information:
- Store in a cool, dry place at controlled room temperature 15-30ºC (59-86ºF)
- Protect from moisture
- Store in original container for better identification
- Use by expiration date on package
Inactive Ingredients:
Microcrystalline Cellulose, Stearic Acid, Colloidal Silicon Dioxide, Magnesium Stearate, Croscarmellose Sodium.
Manufactured for/Distributed by:
Marlex Pharmaceuticals, Inc.,
New Castle, DE 19720
Rev.1 6/20 GP