Label: BENZETHONIUM CHLORIDE PLUS DYCLONINE HYDROCHLORIDE- liquid bandage liquid
- NDC Code(s): 68016-615-00
- Packager: Chain Drug Consortium, LLC
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated February 2, 2024
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredients
- Purpose
- Uses
- Warnings
- Directions
- Other information
- Inactive ingredients
- Questions
-
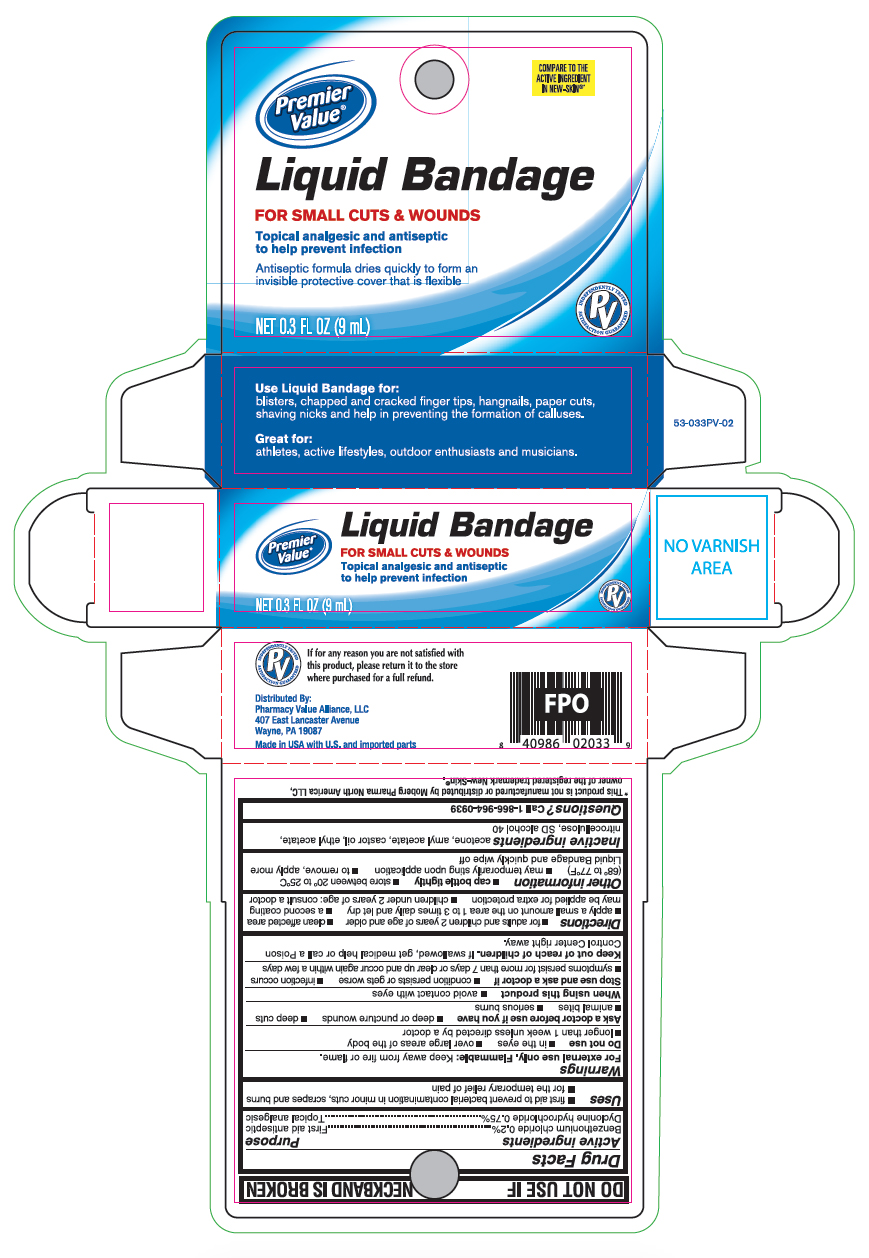
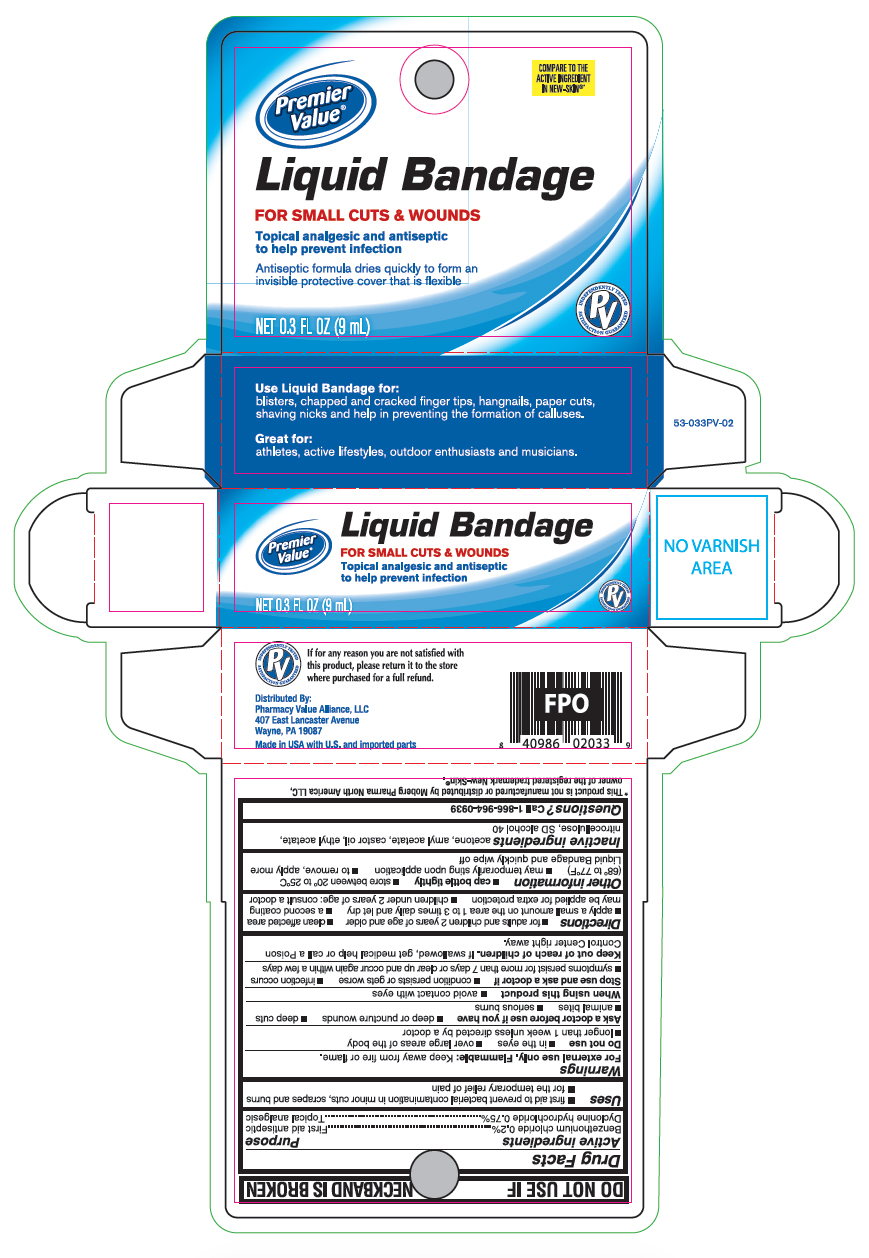
Principal Display Panel
Premier Value
Liquid Bandage
FOR SMALL CUTS & WOUNDS
Topical analgesic and antiseptic to help prevent infection
Antiseptic formula dries quickly to form an invisible protective cover that is flexible
0.3 FL OZ (9mL)
Use Liquid Bandage for:
blisters, chapped and cracked finger tips, hangnails, paper cuts, shaving nicks and help in preventing the formation of calluses.
Great for:
athletes, active lifestyles, outdoor enthusiasts and musicians.

-
INGREDIENTS AND APPEARANCE
BENZETHONIUM CHLORIDE PLUS DYCLONINE HYDROCHLORIDE
liquid bandage liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:68016-615 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZETHONIUM CHLORIDE (UNII: PH41D05744) (BENZETHONIUM - UNII:1VU15B70BP) BENZETHONIUM CHLORIDE 0.2 mg in 9 mL DYCLONINE HYDROCHLORIDE (UNII: ZEC193879Q) (DYCLONINE - UNII:078A24Q30O) DYCLONINE HYDROCHLORIDE 0.75 mg in 9 mL Inactive Ingredients Ingredient Name Strength ACETONE (UNII: 1364PS73AF) AMYL ACETATE (UNII: 92Q24NH7AS) CASTOR OIL (UNII: D5340Y2I9G) ETHYL ACETATE (UNII: 76845O8NMZ) PYROXYLIN (UNII: KYR8BR2X6O) ALCOHOL (UNII: 3K9958V90M) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:68016-615-00 9 mL in 1 BOTTLE, WITH APPLICATOR; Type 0: Not a Combination Product 12/07/2005 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M003 12/07/2005 Labeler - Chain Drug Consortium, LLC (101668460)