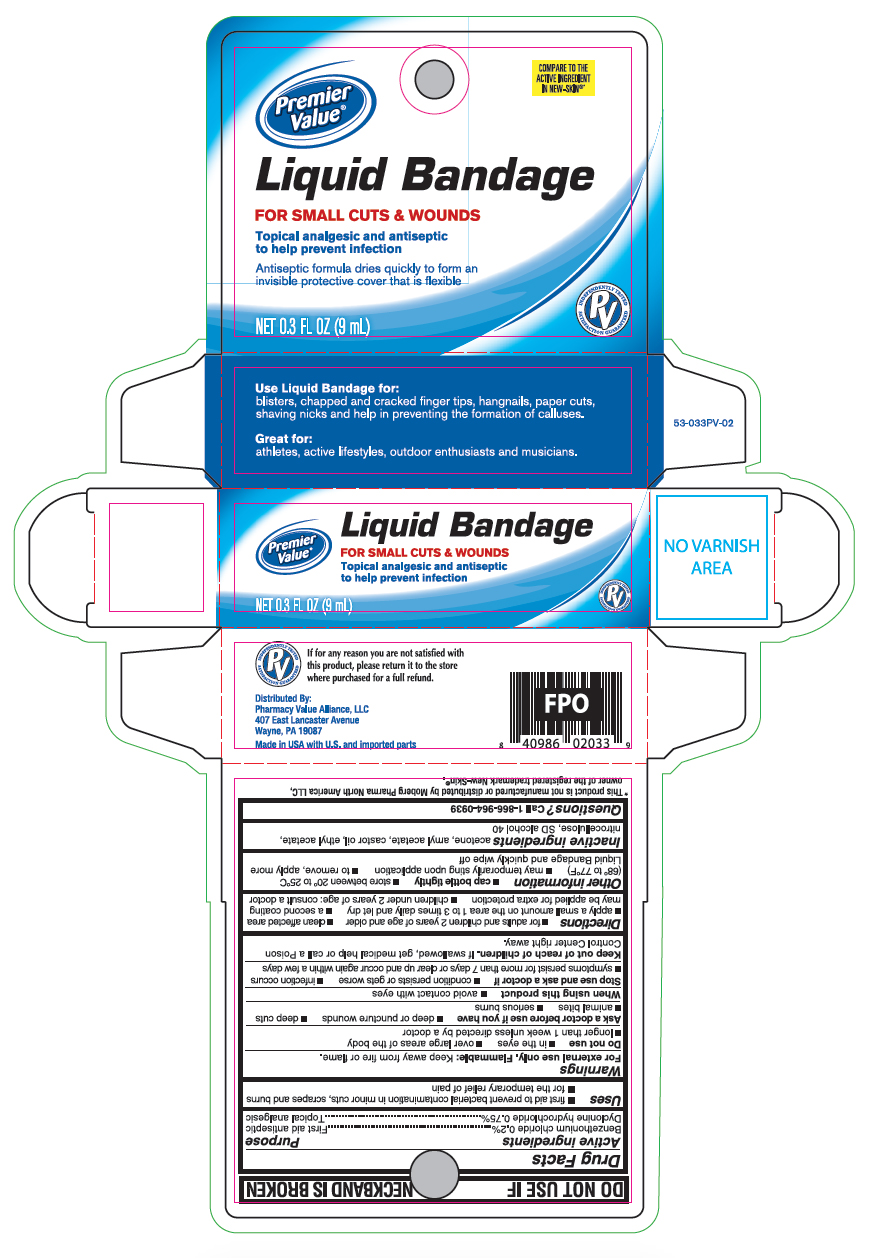
Uses
- first aid to prevent bacterial contamination in minor cuts, scrapes and burns
- for the temporary relief of pain
Warnings
For external use only. Flammable: Keep away from fire or flame.
Directions
- for adults and children 2 years of age and older
- clean affected area
- apply a small amount on the area 1 to 3 times daily and let dry
- a second coating may be applied for extra protection
- children under 2 years of age; consult a doctor
Other information
- cap bottle tightly
- store between 20° to 25°C (68° to 77°F)
- may temporarily sting upon application
- to remove, apply more Liquid Bandage and quickly wipe off
Inactive ingredients
acetone, amyl acetate, castor oil, ethyl acetate, nitrocellulose, SD slcohol 40
Principal Display Panel
Premier Value
Liquid Bandage
FOR SMALL CUTS & WOUNDS
Topical analgesic and antiseptic to help prevent infection
Antiseptic formula dries quickly to form an invisible protective cover that is flexible
0.3 FL OZ (9mL)
Use Liquid Bandage for:
blisters, chapped and cracked finger tips, hangnails, paper cuts, shaving nicks and help in preventing the formation of calluses.
Great for:
athletes, active lifestyles, outdoor enthusiasts and musicians.