Label: CERAMIDE EYEWISH EYE SUNSCREEN SPF 10- octinoxate and oxybenzone cream
-
Contains inactivated NDC Code(s)
NDC Code(s): 67938-0772-1, 67938-0772-2 - Packager: Elizabeth Arden, Inc
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated October 22, 2012
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- DESCRIPTION
- INDICATIONS AND USAGE
-
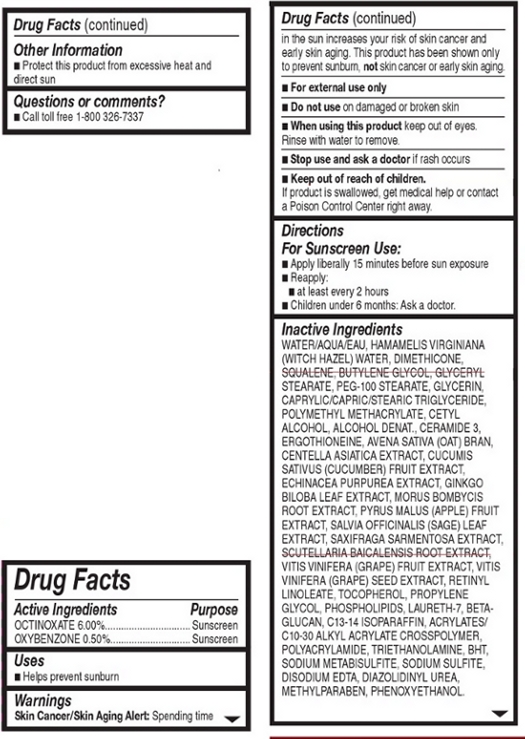
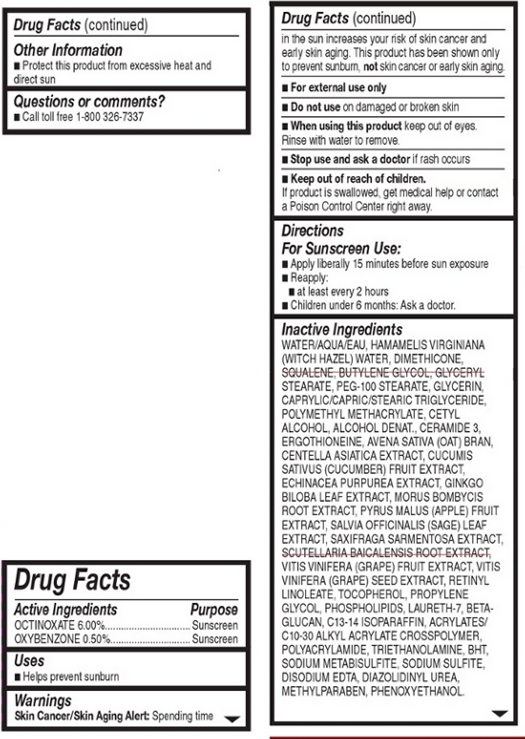
WARNINGS
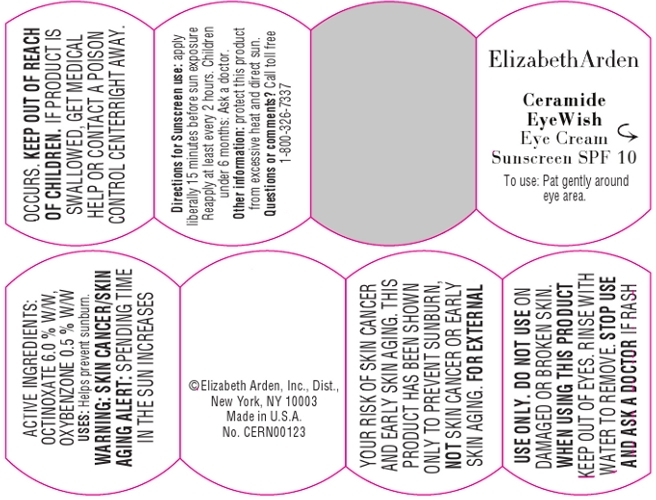
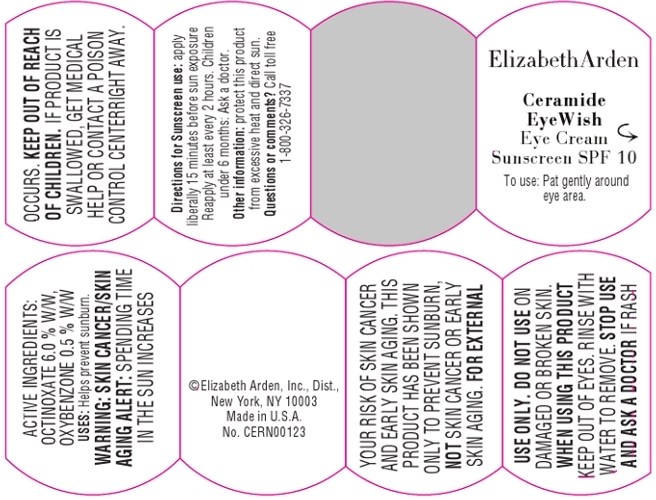
Warnings:
Skin Cancer/Skin Aging Alert: Spending time in the sun increases your risk of skin cancer and early skin aging. This product has been show to prevent sunburn, not skin cancer or early skin aging.
For external use only.
Do not use on damaged or broken skin.
When using this product, keep out of eyes. Rinse with water to remove.
Stop use and ask a doctor if rash occurs.
- OTC - ACTIVE INGREDIENT
-
INACTIVE INGREDIENT
Other Ingredients: Water, Hamamelis Virginiana (Witch Hazel) Water, Dimethicone, Squalene, Butylene Glycol, Glyceryl Stearate, PEG-100 Stearate, Glycerin, Caprylic/Capric/Stearic Triglyceride, Polymethyl Methacrylate, Cetyl Alcohol, Alcohol Denat., Ceramide 3, Ergothioneine, Avena Sativa (Oat) Bran, Centella Asiatica Extract, Cucumin Sativus (Cucumber) Fruit Extract, Echinacea Purpurea Extract, Ginkgo Biloba Leaf Extract, Morus Bombycis Root Extract, Pyrus Malus (Apple) Fruit Extract, Salvia Officinalis (Sage) Leaf Extract, Scutellaria Baicalensis Root Extract, Vitis Vinifera (Grape) Fruit Extratct, Vitin Vinifera (Grape) Seed Extract, Retinyl Linoleate, Tocopherol, Propylene Glycol, Phospholipids, Laureth-7, Beta-Glucan, C13-14 Isoparaffin, Acrylates/C10-30 Alkyl Acrylate Crosspolymer, Polyacrylamide, Triethanolamine, BHT, Sodium Metabisulfite, Sodium Sulfite, Disodium EDTA, Diazolidnyl Urea, Methyl Paraben, Phenoxyethanol.
- DOSAGE & ADMINISTRATION
- OTC - KEEP OUT OF REACH OF CHILDREN
- OTC - PURPOSE
- OTC - WHEN USING
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
CERAMIDE EYEWISH EYE SUNSCREEN SPF 10
octinoxate and oxybenzone creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:67938-0772 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 0.852 g in 14.2 g OXYBENZONE (UNII: 95OOS7VE0Y) (OXYBENZONE - UNII:95OOS7VE0Y) OXYBENZONE 0.071 g in 14.2 g Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) HAMAMELIS VIRGINIANA LEAF WATER (UNII: 8FP93ED6H2) DIMETHICONE (UNII: 92RU3N3Y1O) SQUALENE (UNII: 7QWM220FJH) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) PEG-100 STEARATE (UNII: YD01N1999R) GLYCERIN (UNII: PDC6A3C0OX) CETYL ALCOHOL (UNII: 936JST6JCN) ALCOHOL (UNII: 3K9958V90M) TROLAMINE (UNII: 9O3K93S3TK) OXYBENZONE (UNII: 95OOS7VE0Y) TOCOPHEROL (UNII: R0ZB2556P8) CARBOMER INTERPOLYMER TYPE A (55000 MPA.S) (UNII: 59TL3WG5CO) POLYACRYLAMIDE (10000 MW) (UNII: E2KR9C9V2I) SAXIFRAGA STOLONIFERA LEAF (UNII: O3TMV4903H) WINE GRAPE (UNII: 3GOV20705G) C13-14 ISOPARAFFIN (UNII: E4F12ROE70) DIAZOLIDINYL UREA (UNII: H5RIZ3MPW4) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) CUCUMBER (UNII: YY7C30VXJT) RETINYL LINOLEATE (UNII: 61911N8D6W) EDETATE DISODIUM (UNII: 7FLD91C86K) OMEGA-3 FATTY ACIDS (UNII: 71M78END5S) LAURETH-7 (UNII: Z95S6G8201) CERAMIDE 3 (UNII: 4370DF050B) SODIUM METABISULFITE (UNII: 4VON5FNS3C) SODIUM SULFITE (UNII: VTK01UQK3G) SALVIA OFFICINALIS FLOWERING TOP (UNII: 48JCS720FN) GINKGO (UNII: 19FUJ2C58T) APPLE (UNII: B423VGH5S9) MORUS AUSTRALIS ROOT (UNII: 1VL55O45RF) SCUTELLARIA BAICALENSIS ROOT (UNII: 7J95K7ID2S) METHYLPARABEN (UNII: A2I8C7HI9T) CENTELLA ASIATICA (UNII: 7M867G6T1U) PHENOXYETHANOL (UNII: HIE492ZZ3T) OAT BRAN (UNII: KQX236OK4U) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) ECHINACEA PURPUREA (UNII: QI7G114Y98) VITIS VINIFERA SEED (UNII: C34U15ICXA) ERGOTHIONEINE (UNII: BDZ3DQM98W) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:67938-0772-1 1 in 1 BOX 1 NDC:67938-0772-2 14.2 g in 1 JAR Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part352 03/16/2006 Labeler - Elizabeth Arden, Inc (849222187)