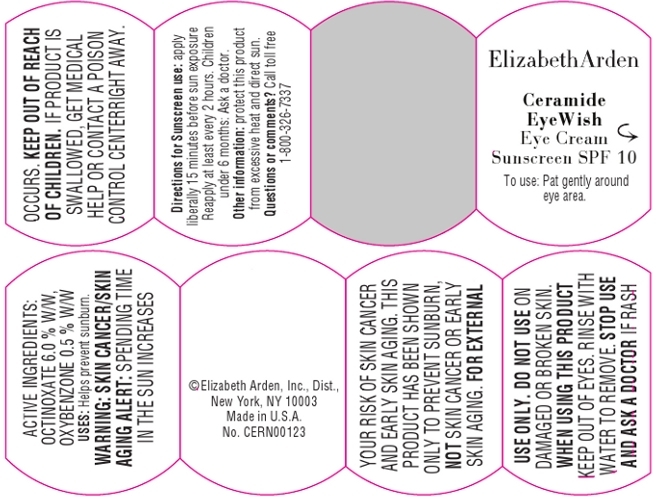
DESCRIPTION
Ceramide and botanical technology combine in one gentle all-around eye cream. This instantly moisturizing formula reduces the appearance of dark circles and fine lines, promotes firmer looking skin, soothes puffiness, and helps protect against future signs of aging due to sun exposure.
INDICATIONS AND USAGE
To Use: Pat gently all around eye area.
For Sunscreen Use: Apply liberally 15 minutes before sun exposure. Reapply at least every 2 hours.
Childre Under 6: Ask a doctor.
WARNINGS
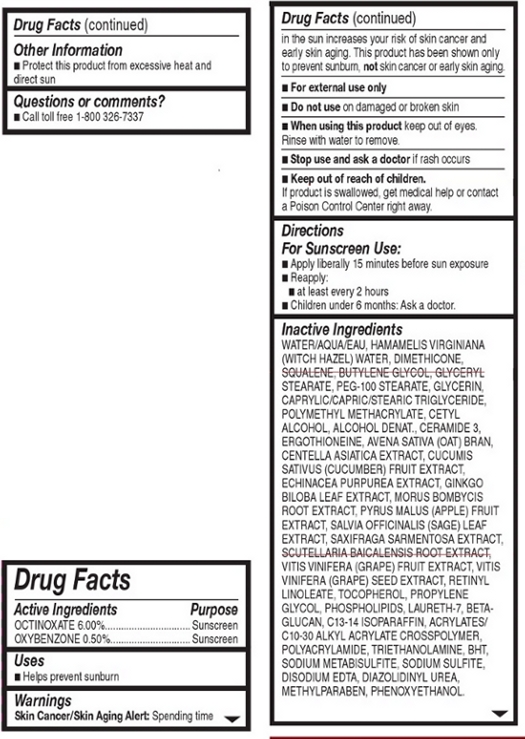
Warnings:
Skin Cancer/Skin Aging Alert: Spending time in the sun increases your risk of skin cancer and early skin aging. This product has been show to prevent sunburn, not skin cancer or early skin aging.
For external use only.
Do not use on damaged or broken skin.
When using this product, keep out of eyes. Rinse with water to remove.
Stop use and ask a doctor if rash occurs.
INACTIVE INGREDIENT
Other Ingredients: Water, Hamamelis Virginiana (Witch Hazel) Water, Dimethicone, Squalene, Butylene Glycol, Glyceryl Stearate, PEG-100 Stearate, Glycerin, Caprylic/Capric/Stearic Triglyceride, Polymethyl Methacrylate, Cetyl Alcohol, Alcohol Denat., Ceramide 3, Ergothioneine, Avena Sativa (Oat) Bran, Centella Asiatica Extract, Cucumin Sativus (Cucumber) Fruit Extract, Echinacea Purpurea Extract, Ginkgo Biloba Leaf Extract, Morus Bombycis Root Extract, Pyrus Malus (Apple) Fruit Extract, Salvia Officinalis (Sage) Leaf Extract, Scutellaria Baicalensis Root Extract, Vitis Vinifera (Grape) Fruit Extratct, Vitin Vinifera (Grape) Seed Extract, Retinyl Linoleate, Tocopherol, Propylene Glycol, Phospholipids, Laureth-7, Beta-Glucan, C13-14 Isoparaffin, Acrylates/C10-30 Alkyl Acrylate Crosspolymer, Polyacrylamide, Triethanolamine, BHT, Sodium Metabisulfite, Sodium Sulfite, Disodium EDTA, Diazolidnyl Urea, Methyl Paraben, Phenoxyethanol.