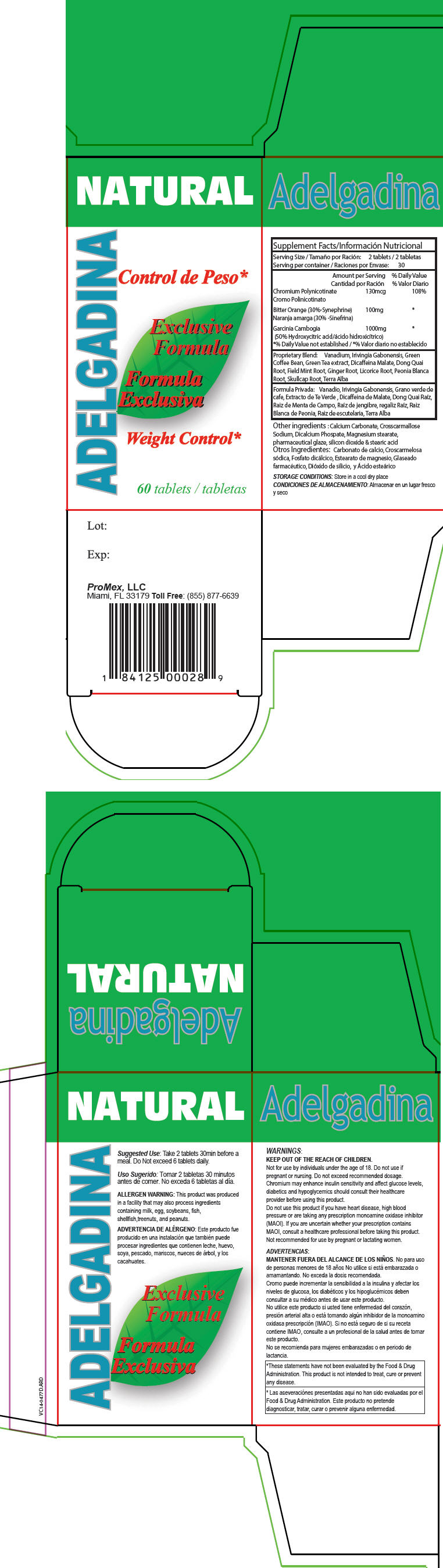
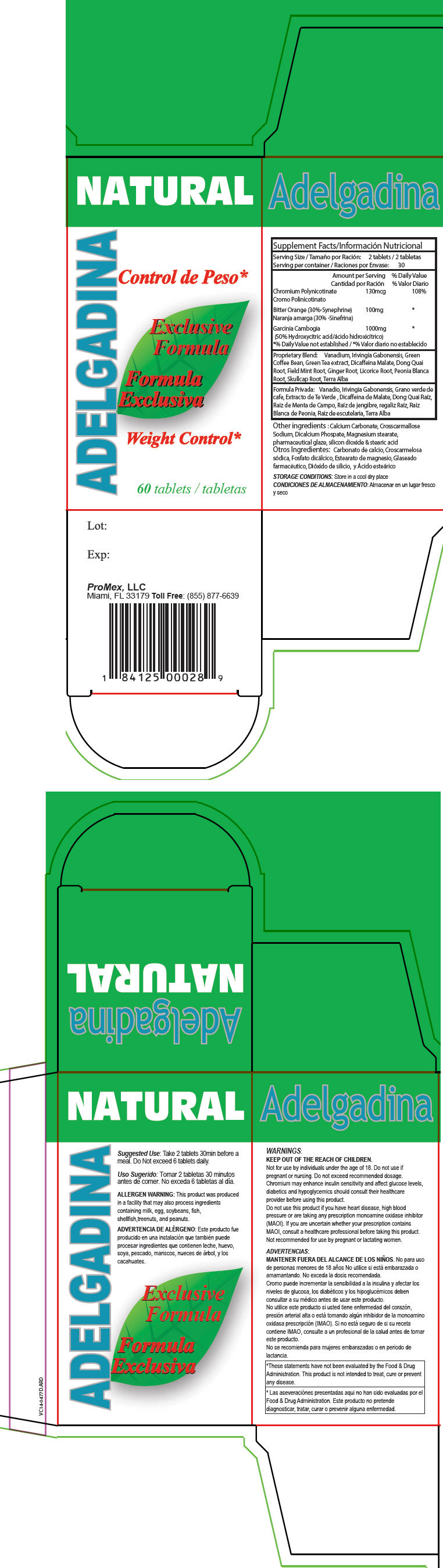
Label: ADELGADINA- chromium nicotinate, citrus aurantium fruit, and garcinia gummi-gutta fruit tablet
- NHRIC Code(s): 58988-1820-1
- Packager: ProMex LLC
- Category: DIETARY SUPPLEMENT
- DEA Schedule: None
- Marketing Status: Dietary Supplement
Drug Label Information
Updated November 23, 2015
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
STATEMENT OF IDENTITY
Supplement Facts Serving Size: 2 tablets Serving per container: 30 Amount per Serving % Daily Value Proprietary Blend: Vanadium, Irivingia Gabonensis, Green Coffee Bean, Green Tea extract, Dicaffeina Malate, Dong Quai Root, Field Mint Root, Ginger Root, Licorice Root, Peonia Blanca Root, Skullcap Root, Terra Alba - *
- % Daily Value not established
Chromium Polynicotinate 130mcg 108% Bitter Orange (30%-Synephrine) 100mg * Garcinia Cambogia
(50% Hydroxycitric acid)1000mg * Other ingredients : Calcium Carbonate, Crosscarmallose Sodium, Dicalcium Phospate, Magnesium stearate, pharmaceutical glaze, silicon dioxide & stearic acid
- STORAGE CONDITIONS
- Suggested Use
- ALLERGEN WARNING
-
WARNINGS
KEEP OUT OF THE REACH OF CHILDREN.
Not for use by individuals under the age of 18. Do not use if pregnant or nursing. Do not exceed recommended dosage. Chromium may enhance insulin sensitivity and affect glucose levels, diabetics and hypoglycemics should consult their healthcare provider before using this product.
Do not use this product if you have heart disease, high blood pressure or are taking any prescription monoamine oxidase inhibitor (MAOI). If you are uncertain whether your prescription contains MAOI, consult a healthcare professional before taking this product. Not recommended for use by pregnant or lactating women.
*These statements have not been evaluated by the Food & Drug Administration. This product is not intended to treat, cure or prevent any disease. - HEALTH CLAIM
- PRINCIPAL DISPLAY PANEL - 60 Tablet Bottle Box
-
INGREDIENTS AND APPEARANCE
ADELGADINA
chromium nicotinate, citrus aurantium fruit, and garcinia gummi-gutta fruit tabletProduct Information Product Type DIETARY SUPPLEMENT Item Code (Source) NHRIC:58988-1820 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CHROMIUM NICOTINATE (UNII: A150AY412V) (NIACIN - UNII:2679MF687A, CHROMIC CATION - UNII:X1N4508KF1) CHROMIUM NICOTINATE 130 ug CITRUS AURANTIUM FRUIT (UNII: DQD16J2B5O) (CITRUS AURANTIUM FRUIT - UNII:DQD16J2B5O) CITRUS AURANTIUM FRUIT 100 mg GARCINIA GUMMI-GUTTA FRUIT (UNII: D0QJI8UQVR) (GARCINIA GUMMI-GUTTA FRUIT - UNII:D0QJI8UQVR) GARCINIA GUMMI-GUTTA FRUIT 1000 mg Inactive Ingredients Ingredient Name Strength VANADIUM (UNII: 00J9J9XKDE) BUSH MANGO (UNII: CAZ59QT0HZ) ARABICA COFFEE BEAN (UNII: 3SW678MX72) GREEN TEA LEAF (UNII: W2ZU1RY8B0) CAFFEINE (UNII: 3G6A5W338E) ANGELICA SINENSIS ROOT (UNII: B66F4574UG) MENTHA ARVENSIS WHOLE (UNII: 46477RRB3O) GINGER (UNII: C5529G5JPQ) LICORICE (UNII: 61ZBX54883) PAEONIA LACTIFLORA ROOT (UNII: 3Z3866YW6P) SCUTELLARIA LATERIFLORA (UNII: 7BP4DH5PDC) CALCIUM SULFATE DIHYDRATE (UNII: 4846Q921YM) CALCIUM CARBONATE (UNII: H0G9379FGK) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) ANHYDROUS DIBASIC CALCIUM PHOSPHATE (UNII: L11K75P92J) MAGNESIUM STEARATE (UNII: 70097M6I30) SHELLAC (UNII: 46N107B71O) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) STEARIC ACID (UNII: 4ELV7Z65AP) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NHRIC:58988-1820-1 1 in 1 BOX 1 60 in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date DIETARY SUPPLEMENT 12/04/1961 Supplement Facts Serving Size : Serving per Container : Amount Per Serving % Daily Value color scoring 1 shape size (solid drugs) 19 mm Labeler - ProMex LLC (789974388)