Label: CLEOCIN T- clindamycin phosphate gel
- NDC Code(s): 0009-1313-01, 0009-1333-01
- Packager: Pharmacia & Upjohn Company LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Export only
Drug Label Information
Updated July 28, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
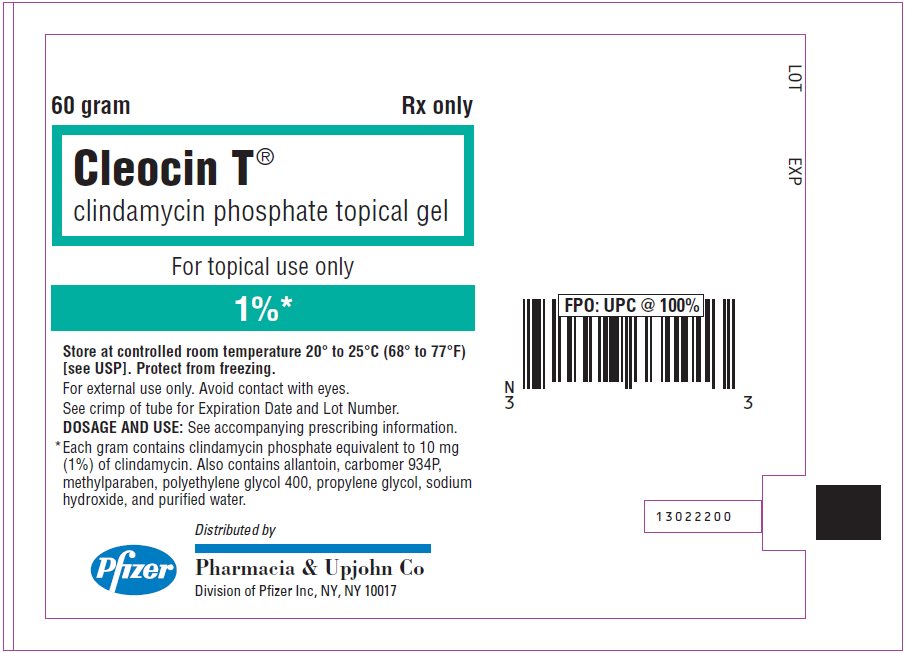
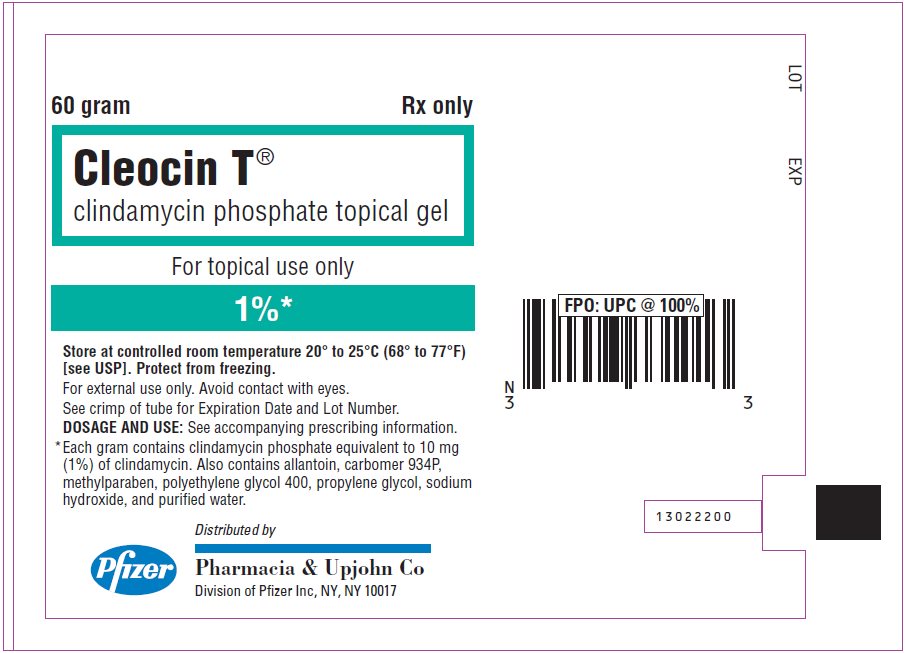
PRINCIPAL DISPLAY PANEL - 60 gram Tube Label
60 gram
Rx only
Cleocin T®
clindamycin phosphate topical gelFor topical use only
1%*
Store at controlled room temperature 20° to 25°C (68° to 77°F)
[see USP]. Protect from freezing.
For external use only. Avoid contact with eyes.
See crimp of tube for Expiration Date and Lot Number.
DOSAGE AND USE: See accompanying prescribing information.
* Each gram contains clindamycin phosphate equivalent to 10 mg
(1%) of clindamycin. Also contains allantoin, carbomer 934P,
methylparaben, polyethylene glycol 400, propylene glycol, sodium
hydroxide, and purified water.Pfizer
Distributed by
Pharmacia & Upjohn Co
Division of Pfizer Inc, NY, NY 10017 - PRINCIPAL DISPLAY PANEL - 60 gram Tube Carton
-
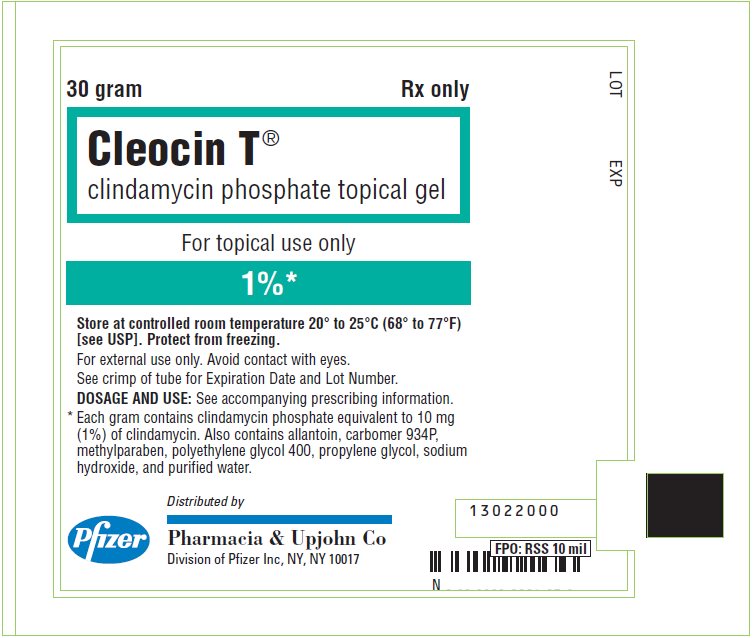
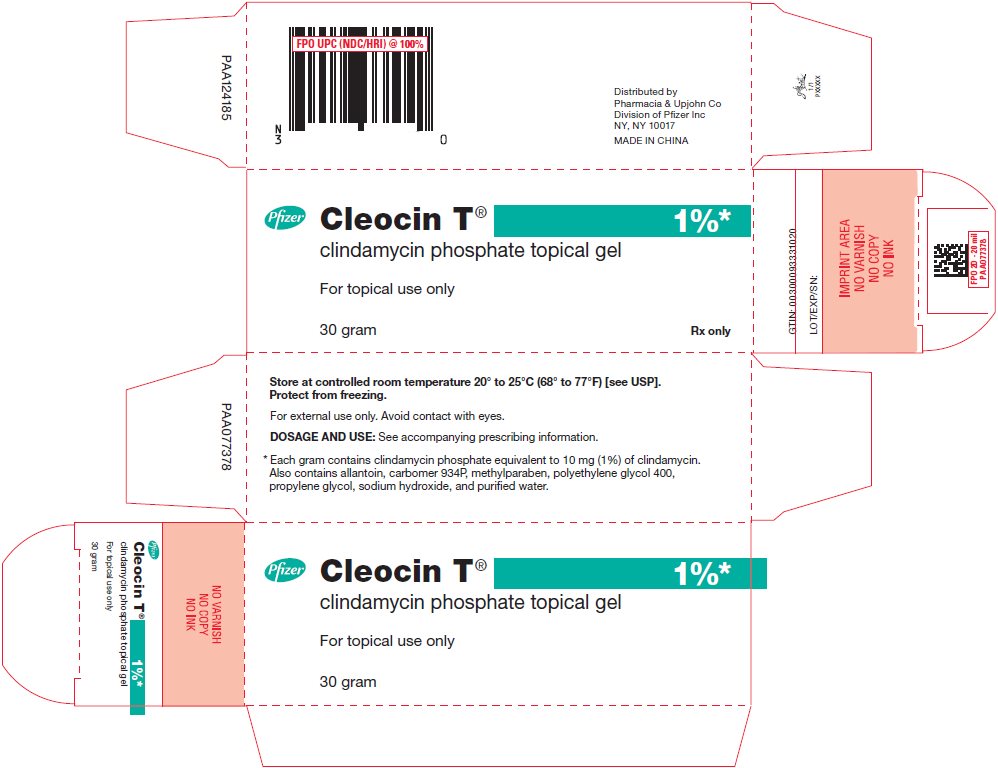
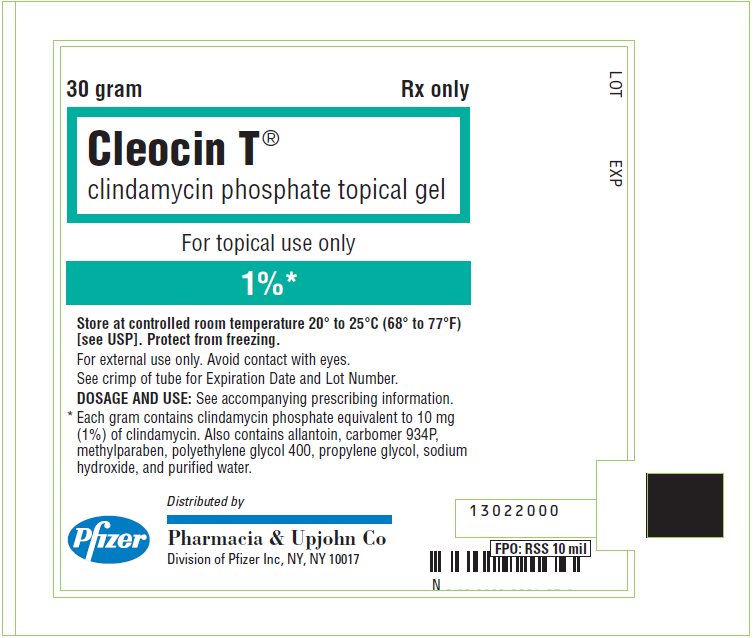
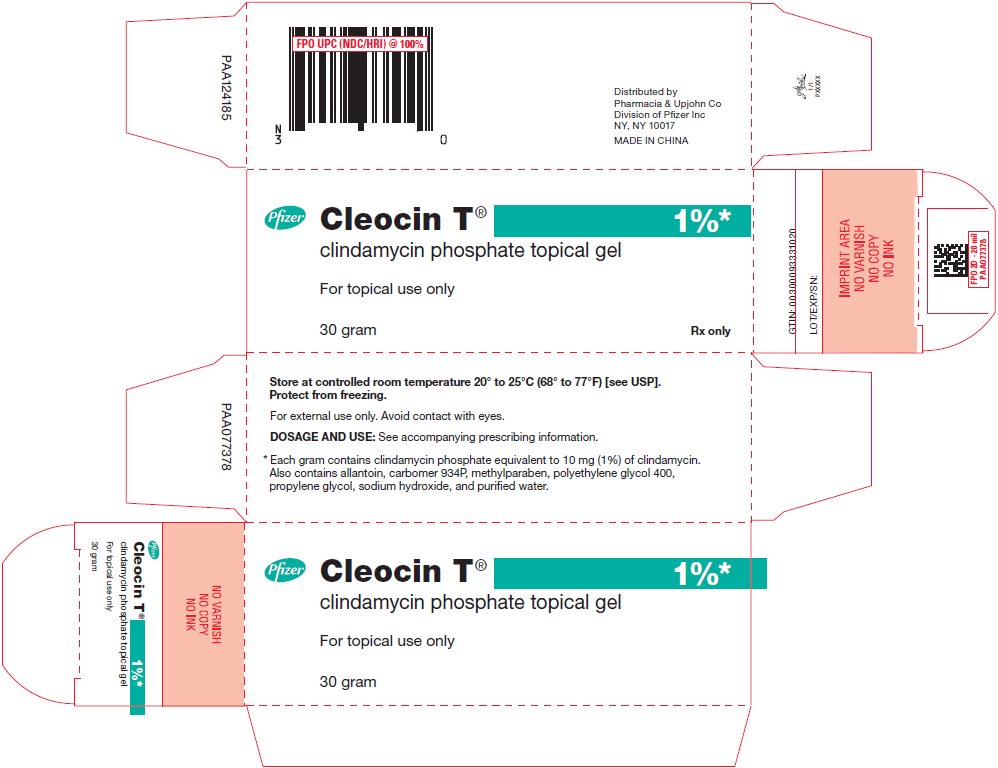
PRINCIPAL DISPLAY PANEL - 30 gram Tube Label
30 gram
Rx only
Cleocin T®
clindamycin phosphate topical gelFor topical use only
1%*
Store at controlled room temperature 20° to 25°C (68° to 77°F)
[see USP]. Protect from freezing.
For external use only. Avoid contact with eyes.
See crimp of tube for Expiration Date and Lot Number.
DOSAGE AND USE: See accompanying prescribing information.
* Each gram contains clindamycin phosphate equivalent to 10 mg
(1%) of clindamycin. Also contains allantoin, carbomer 934P,
methylparaben, polyethylene glycol 400, propylene glycol, sodium
hydroxide, and purified water.Pfizer
Distributed by
Pharmacia & Upjohn Co
Division of Pfizer Inc, NY, NY 10017 - PRINCIPAL DISPLAY PANEL - 30 gram Tube Carton
-
INGREDIENTS AND APPEARANCE
CLEOCIN T
clindamycin phosphate gelProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0009-1333 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CLINDAMYCIN PHOSPHATE (UNII: EH6D7113I8) (CLINDAMYCIN - UNII:3U02EL437C) CLINDAMYCIN 10 mg in 1 g Inactive Ingredients Ingredient Name Strength CARBOMER HOMOPOLYMER TYPE B (ALLYL PENTAERYTHRITOL CROSSLINKED) (UNII: HHT01ZNK31) ALLANTOIN (UNII: 344S277G0Z) METHYLPARABEN (UNII: A2I8C7HI9T) POLYETHYLENE GLYCOL 400 (UNII: B697894SGQ) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) SODIUM HYDROXIDE (UNII: 55X04QC32I) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0009-1333-01 1 in 1 CARTON 11/01/2021 1 30 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Export only 11/01/2021 CLEOCIN T
clindamycin phosphate gelProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0009-1313 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CLINDAMYCIN PHOSPHATE (UNII: EH6D7113I8) (CLINDAMYCIN - UNII:3U02EL437C) CLINDAMYCIN 10 mg in 1 g Inactive Ingredients Ingredient Name Strength CARBOMER HOMOPOLYMER TYPE B (ALLYL PENTAERYTHRITOL CROSSLINKED) (UNII: HHT01ZNK31) ALLANTOIN (UNII: 344S277G0Z) METHYLPARABEN (UNII: A2I8C7HI9T) POLYETHYLENE GLYCOL 400 (UNII: B697894SGQ) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) SODIUM HYDROXIDE (UNII: 55X04QC32I) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0009-1313-01 1 in 1 CARTON 11/01/2021 1 60 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Export only 11/01/2021 Labeler - Pharmacia & Upjohn Company LLC (618054084) Establishment Name Address ID/FEI Business Operations Pharmacia & Upjohn Company LLC 618054084 MANUFACTURE(0009-1333, 0009-1313) , ANALYSIS(0009-1333, 0009-1313) , PACK(0009-1333, 0009-1313) , LABEL(0009-1333, 0009-1313)