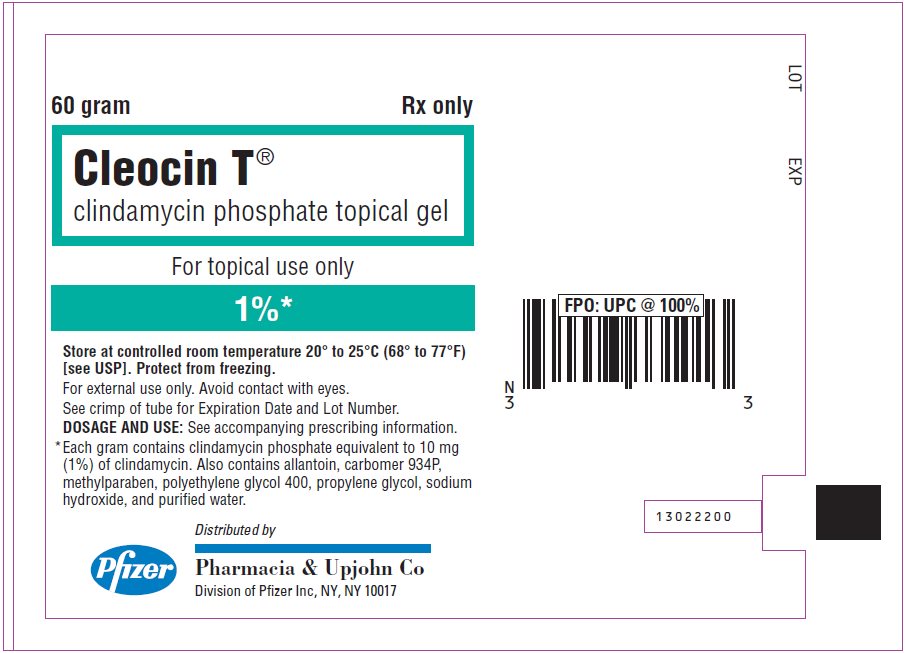
PRINCIPAL DISPLAY PANEL - 60 gram Tube Label
60 gram
Rx only
Cleocin T®
clindamycin phosphate topical gel
For topical use only
1%*
Store at controlled room temperature 20° to 25°C (68° to 77°F)
[see USP]. Protect from freezing.
For external use only. Avoid contact with eyes.
See crimp of tube for Expiration Date and Lot Number.
DOSAGE AND USE: See accompanying prescribing information.
* Each gram contains clindamycin phosphate equivalent to 10 mg
(1%) of clindamycin. Also contains allantoin, carbomer 934P,
methylparaben, polyethylene glycol 400, propylene glycol, sodium
hydroxide, and purified water.
Pfizer
Distributed by
Pharmacia & Upjohn Co
Division of Pfizer Inc, NY, NY 10017
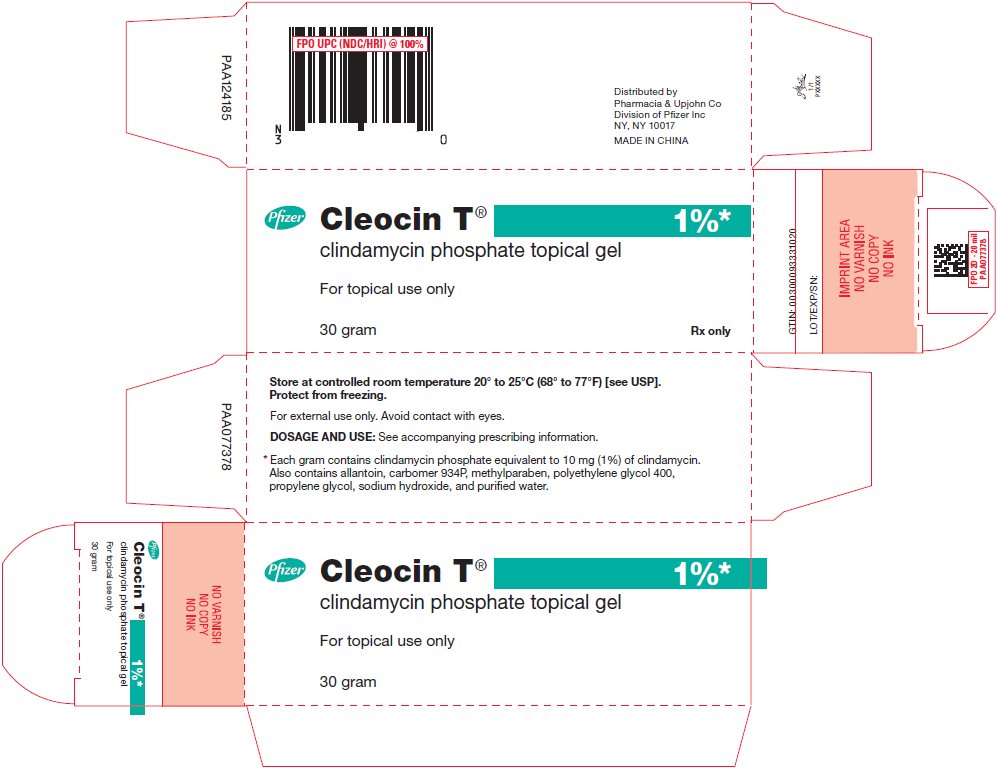
PRINCIPAL DISPLAY PANEL - 60 gram Tube Carton
Pfizer
Cleocin T®
1%*
clindamycin phosphate topical gel
For topical use only
60 gram
Rx only
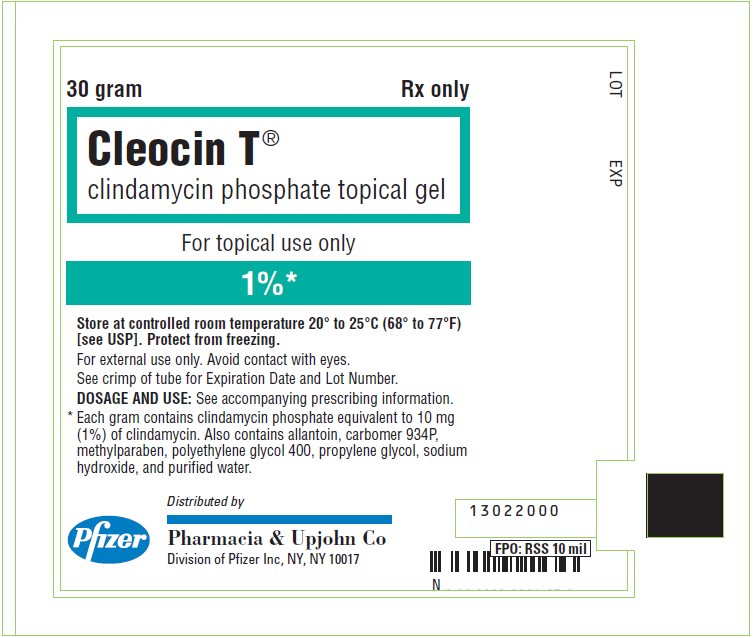
PRINCIPAL DISPLAY PANEL - 30 gram Tube Label
30 gram
Rx only
Cleocin T®
clindamycin phosphate topical gel
For topical use only
1%*
Store at controlled room temperature 20° to 25°C (68° to 77°F)
[see USP]. Protect from freezing.
For external use only. Avoid contact with eyes.
See crimp of tube for Expiration Date and Lot Number.
DOSAGE AND USE: See accompanying prescribing information.
* Each gram contains clindamycin phosphate equivalent to 10 mg
(1%) of clindamycin. Also contains allantoin, carbomer 934P,
methylparaben, polyethylene glycol 400, propylene glycol, sodium
hydroxide, and purified water.
Pfizer
Distributed by
Pharmacia & Upjohn Co
Division of Pfizer Inc, NY, NY 10017