Label: RITE AID EXTRA STRENGTH- aluminum hydroxide and magnesium carbonate tablet, chewable
- NDC Code(s): 11822-1050-0
- Packager: RITE AID CORPORATION
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated November 17, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
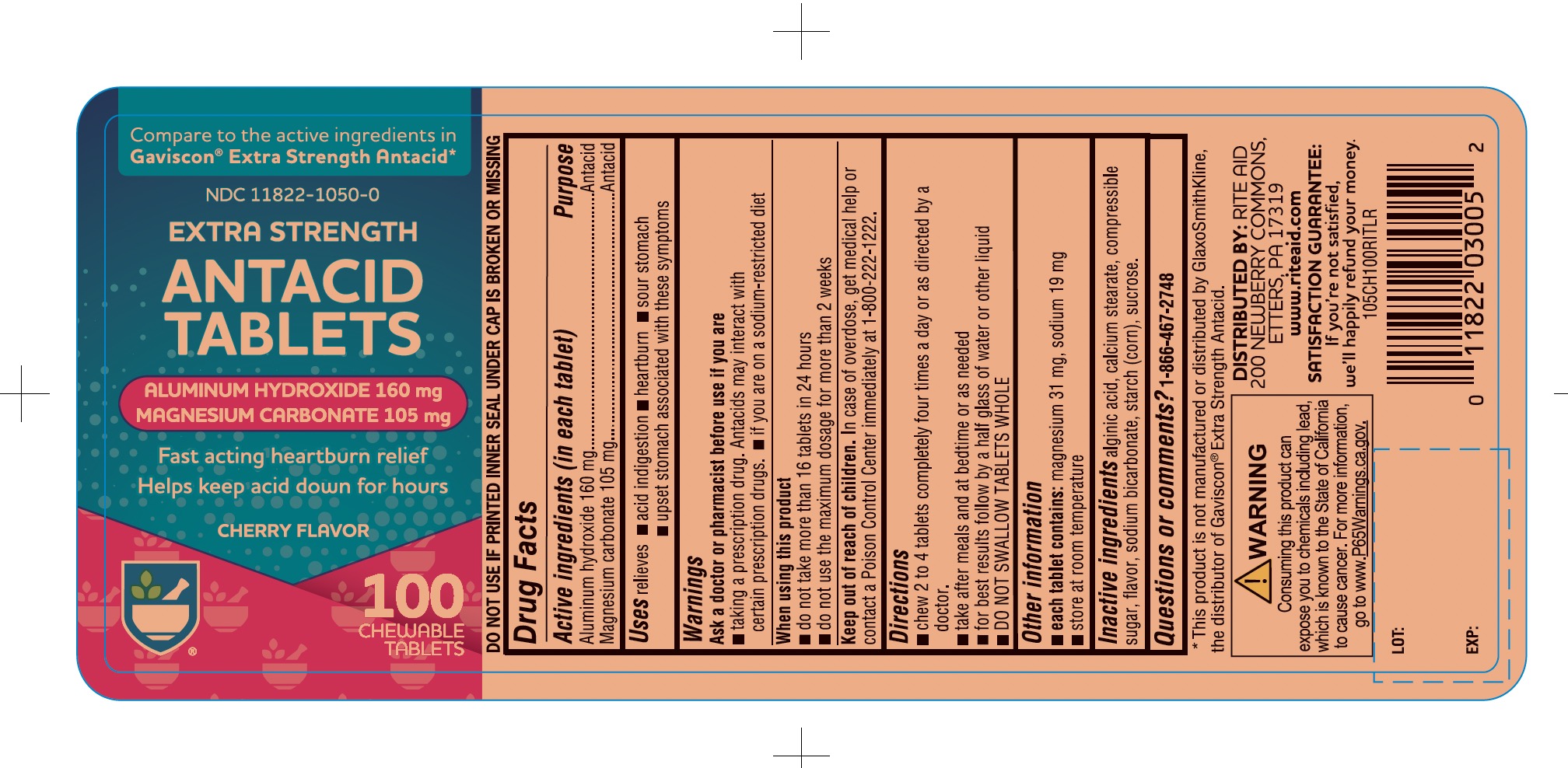
- Active ingredients (in each tablet)
- Purpose
- Uses
- Warnings
- Directions
- Other information
- Inactive ingredients
-
Principal Display Panel
RITE AID PHARMACY®
NDC 11822-1050-0
Compare to the active ingredients Gaviscon® Extra Strength Antacid
Extra Strength
Antacid Tablets
Aluminum Hydroxide
Magnesium Carbonate
- •
- Fast-Acting Heartburn Relief
- •
- Helps Keep Acid Down for Hours
Cherry Flavor
100 Chewable Tablets
Distributed by:
*This product is not manufactured or distributed by GlaxoSmithKline, the distributor of Gaviscon® Extra Strength Antacid.
WARNING: Consuming this product can expose you to chemicals including lead, which is known to the State of California, go to www.P65Warnings.ca.gov.
-
INGREDIENTS AND APPEARANCE
RITE AID EXTRA STRENGTH
aluminum hydroxide and magnesium carbonate tablet, chewableProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:11822-1050 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) (ALUMINUM HYDROXIDE - UNII:5QB0T2IUN0) ALUMINUM HYDROXIDE 160 mg MAGNESIUM CARBONATE (UNII: 0E53J927NA) (CARBONATE ION - UNII:7UJQ5OPE7D) MAGNESIUM CARBONATE 105 mg Inactive Ingredients Ingredient Name Strength ALGINIC ACID (UNII: 8C3Z4148WZ) CALCIUM STEARATE (UNII: 776XM7047L) CORN SYRUP (UNII: 9G5L16BK6N) SODIUM BICARBONATE (UNII: 8MDF5V39QO) STARCH, CORN (UNII: O8232NY3SJ) SUCROSE (UNII: C151H8M554) Product Characteristics Color WHITE Score no score Shape ROUND Size 13mm Flavor CHERRY Imprint Code RP105 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:11822-1050-0 100 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 12/13/2022 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M001 12/13/2022 Labeler - RITE AID CORPORATION (014578892)