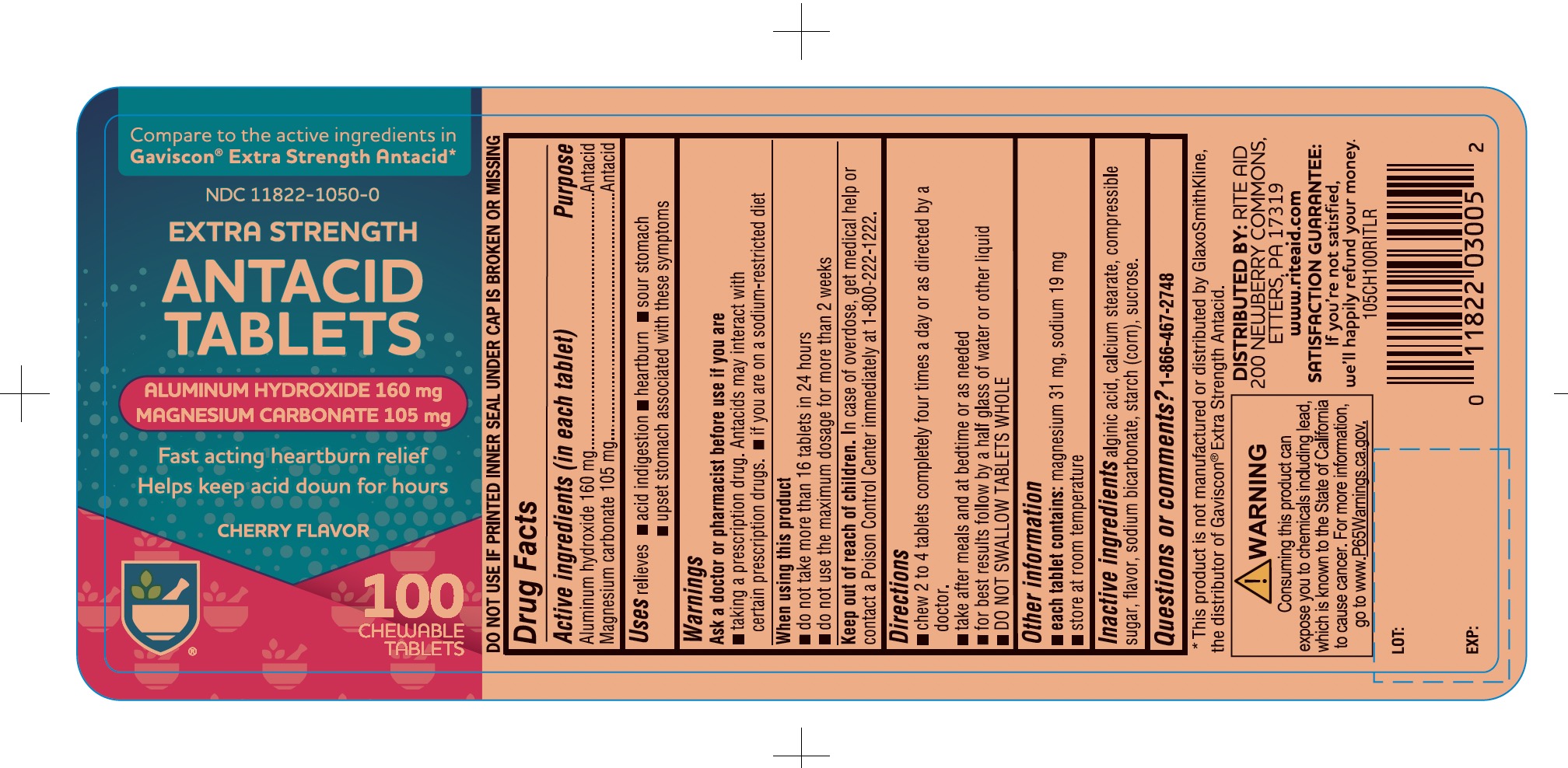
Uses
relieves
- •
- acid indigestion
- •
- heartburn
- •
- sour stomach
- •
- upset stomach associated with these symptoms
Warnings
Ask a doctor or pharmacist before use if you are
- •
- taking a prescription drug. Antacids may interact with certain prescription drugs
- •
- If you are on a sodium-restricted diet
Directions
- •
- chew 2 to 4 tablets completely four times a day or as directed by a doctor.
- •
- take after meals and at bedtime or as needed
- •
- for best results follow by a half glass of water or other liquid
- •
- DO NOT SWALLOW TABLETS WHOLE
Other information
- •
- each tablet contains: magnesium 31 mg, sodium 19 mg,
- •
- Store at room temperature.
Inactive ingredients
alginic acid, calcium stearate, compressible sugar, flavor, sodium bicarbonate, starch (corn), sucrose.
Principal Display Panel
RITE AID PHARMACY®
NDC 11822-1050-0
Compare to the active ingredients Gaviscon® Extra Strength Antacid
Extra Strength
Antacid Tablets
Aluminum Hydroxide
Magnesium Carbonate
- •
- Fast-Acting Heartburn Relief
- •
- Helps Keep Acid Down for Hours
Cherry Flavor
100 Chewable Tablets
Distributed by:
*This product is not manufactured or distributed by GlaxoSmithKline, the distributor of Gaviscon® Extra Strength Antacid.
WARNING: Consuming this product can expose you to chemicals including lead, which is known to the State of California, go to www.P65Warnings.ca.gov.