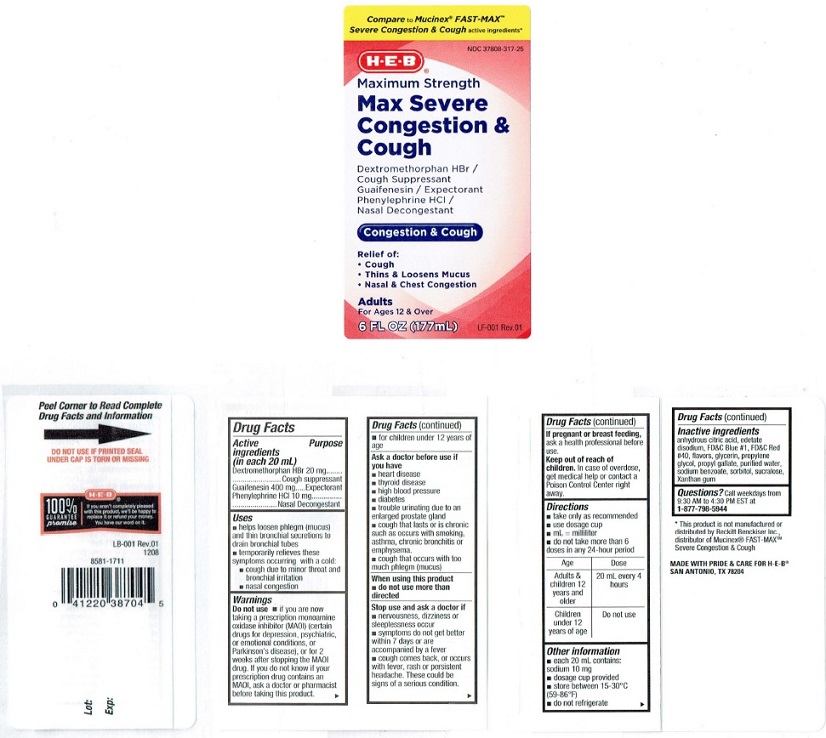
Label: H E B MAX SEVERE CONGESTION AND COUGH MAXIUM STRENGTH- dextromethorphan hydrobromide, guaifenesin, phenylephrine hydrochloride liquid
- NDC Code(s): 37808-317-25
- Packager: H E B
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated December 29, 2023
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
-
WARNINGS
Warnings
Do not use • if you are now
taking a prescription monoamine
oxiclase inhibitor (MAOI) (certain
drugs for depression. psychiatric.
or emotional conditions, or
Parkinson's disease), or for 2
weeks after stopping the MAOI
drug. If you do not know if your
prescription drug contains an
MAOI, ask a doctor or pharmacist
before taking this product.
• for children under 12 years of
age - ASK DOCTOR
- WHEN USING
- STOP USE
- If pregnant or breast feeding,
- KEEP OUT OF REACH OF CHILDREN
- DOSAGE & ADMINISTRATION
- OTHER SAFETY INFORMATION
- INACTIVE INGREDIENT
- QUESTIONS
-
Product Label
Compare to Mucinex® FAST-MAX™
Severe Congestion and Cough active ingredients*
NDC 37808-317-25
H-E-B®Maximum Strength
Max Severe
Congestion &
Cough
Dextromethorphan HBr /
Cough Suppressant
Guaifenesin / Expectorant
Phenylephrine HCI /
Nasal DecongestantCongestion & Cough
Relief of:
• Cough
• Thins & Loosens Mucus
• Nasal & Chest CongestionAdults
For Ages 12 & Over
6 FL OZ (177mL)
LF 001 Rev.01
MADE WITH PRIDE AND CARE FOR H-E-B®
SAN ANTONIO, TEXAS, 782044
Peel Corner to Read Complete
Drug Facts and Information →DO NOT USE IF PRINTED SEAL
UNDER CAP IS TORN OR MISSING
H-E-B®
100%
GUARANTEE
promiseIf you aren't completely pleased
with this product, we'll be happy to
replace it or refund your money.
You have our word on it.
LB·001 Rev.01
1208
8581-17110 41220 38704 5
Lot:
Exp:*This product is not manufactured or
distributed by Reckill Benckiser Inc..
distributor of Mucinex® FAST-MAXTM
Severe Congeslion &CoughMADE WITH PRIDE & CARE FOR H·E-B®
SAN ANTONIO. TX 78204
res
-
INGREDIENTS AND APPEARANCE
H E B MAX SEVERE CONGESTION AND COUGH MAXIUM STRENGTH
dextromethorphan hydrobromide, guaifenesin, phenylephrine hydrochloride liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:37808-317 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DEXTROMETHORPHAN HYDROBROMIDE (UNII: 9D2RTI9KYH) (DEXTROMETHORPHAN - UNII:7355X3ROTS) DEXTROMETHORPHAN HYDROBROMIDE 20 mg in 20 mL GUAIFENESIN (UNII: 495W7451VQ) (GUAIFENESIN - UNII:495W7451VQ) GUAIFENESIN 400 mg in 20 mL PHENYLEPHRINE HYDROCHLORIDE (UNII: 04JA59TNSJ) (PHENYLEPHRINE - UNII:1WS297W6MV) PHENYLEPHRINE HYDROCHLORIDE 10 mg in 20 mL Inactive Ingredients Ingredient Name Strength ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) EDETATE DISODIUM (UNII: 7FLD91C86K) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) FD&C RED NO. 40 (UNII: WZB9127XOA) GLYCERIN (UNII: PDC6A3C0OX) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) PROPYL GALLATE (UNII: 8D4SNN7V92) WATER (UNII: 059QF0KO0R) SODIUM BENZOATE (UNII: OJ245FE5EU) SORBITOL (UNII: 506T60A25R) SUCRALOSE (UNII: 96K6UQ3ZD4) XANTHAN GUM (UNII: TTV12P4NEE) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:37808-317-25 177 mL in 1 BOTTLE; Type 0: Not a Combination Product 09/14/2012 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M012 09/14/2012 Labeler - H E B (007924756) Registrant - AptaPharma Inc. (790523323) Establishment Name Address ID/FEI Business Operations AptaPharma Inc. 790523323 manufacture(37808-317)