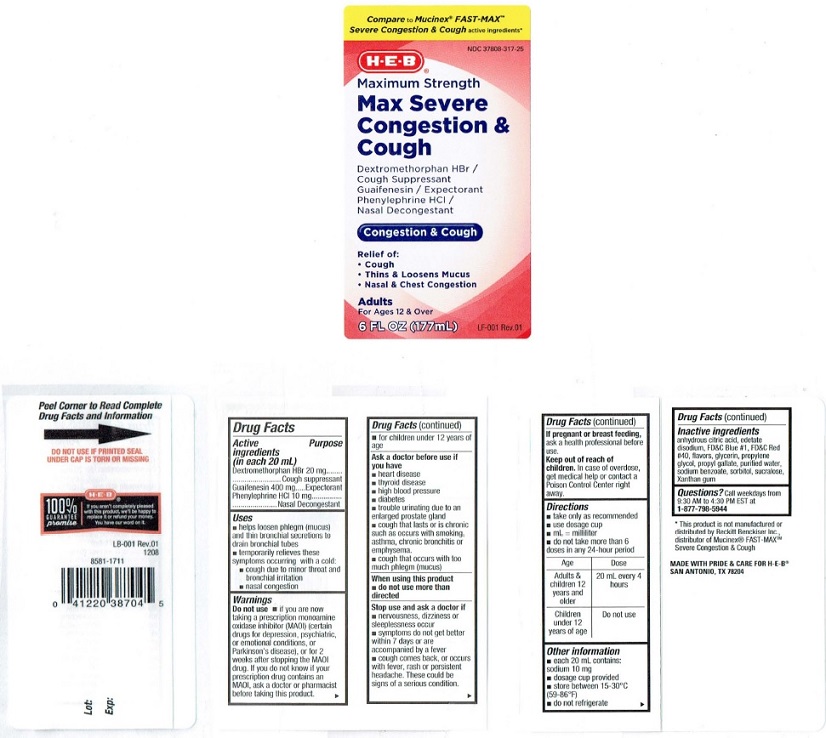
Active ingredients (in each 20 mL)
Dextromethorphan HBr 20 mg
Guaifenesin 400 mg
Phenylephrine HCL 10 mg
Purpose
Dextromethorphan HBr ... Cough suppressant
Guaifenesin ....................Expectorant
Phenylephrine HCL ..........Nasal Decongestant
Uses
• helps loosen phlegm (mucus)
and thin bronchial secretions to
drain bronchial tubes
• temporarily relieves these
symploms occurring with a cold:
• cough due to minor throat and
bronchial irritation
• nasal congestion
Warnings
Do not use • if you are now
taking a prescription monoamine
oxiclase inhibitor (MAOI) (certain
drugs for depression. psychiatric.
or emotional conditions, or
Parkinson's disease), or for 2
weeks after stopping the MAOI
drug. If you do not know if your
prescription drug contains an
MAOI, ask a doctor or pharmacist
before taking this product.
• for children under 12 years of
age
Ask a doctor before use if
you have
• heart disease
• thyroid disease
• high blood pressure
• diabetes
• trouble urinating due to an
enlarged prostate gland
• cough that lasts or is chronic
such as occurs with smoking,
asthma, chronic bronchitis or
emphysema.
• cough that occurs with too
much phlegm (mucus)
Stop use and ask a doctor if
• nervousness, dizziness or
sleeplessness occur
• symptoms do not get better
within 7 days or are
accompanied by a fever
• cough comes back, or occurs
with fever, rash or persistent
headache. These could be
signs of a serious condition.
Keep out of reach of
children. In case of overdose,
get medical help or contact a
Poison Control Center right
away.
Directions
• take only as recommended
• use dosage cup
• mL = milliliter
• do not take more than 6
doses in any 24-hour period
Age Dose
Adults & 20 ml every 4
children 12 hours
years and
older
Children Do not use
under 12
years of age
Other information
- each 20 mL contains: sodium 10 mg
- dosage cup provided
- store between 15-30° C (59-86° F)
- do not refrigerate
Inactive ingredients
anhydrous cilric acid, edetate
disodium, FD&C Blue #1, FD&C Red
#40. flavors. glycerin, propylene
glycol, propyl gallate, purified water,
sodium benzoate, sorbitol, sucralose,
Xanthan gum
Product Label
Compare to Mucinex® FAST-MAX™
Severe Congestion and Cough active ingredients*
NDC 37808-317-25
H-E-B®
Maximum Strength
Max Severe
Congestion &
Cough
Dextromethorphan HBr /
Cough Suppressant
Guaifenesin / Expectorant
Phenylephrine HCI /
Nasal Decongestant
Congestion & Cough
Relief of:
• Cough
• Thins & Loosens Mucus
• Nasal & Chest Congestion
Adults
For Ages 12 & Over
6 FL OZ (177mL)
LF 001 Rev.01
MADE WITH PRIDE AND CARE FOR H-E-B®
SAN ANTONIO, TEXAS, 782044
Peel Corner to Read Complete
Drug Facts and Information →
DO NOT USE IF PRINTED SEAL
UNDER CAP IS TORN OR MISSING
H-E-B®
100%
GUARANTEE
promise
If you aren't completely pleased
with this product, we'll be happy to
replace it or refund your money.
You have our word on it.
LB·001 Rev.01
1208
8581-1711
0 41220 38704 5
Lot:
Exp:
*This product is not manufactured or
distributed by Reckill Benckiser Inc..
distributor of Mucinex® FAST-MAXTM
Severe Congeslion &Cough
MADE WITH PRIDE & CARE FOR H·E-B®
SAN ANTONIO. TX 78204
res