Label: CYCLE- menthol patch
- NDC Code(s): 72587-001-02
- Packager: La Mend, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 7, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
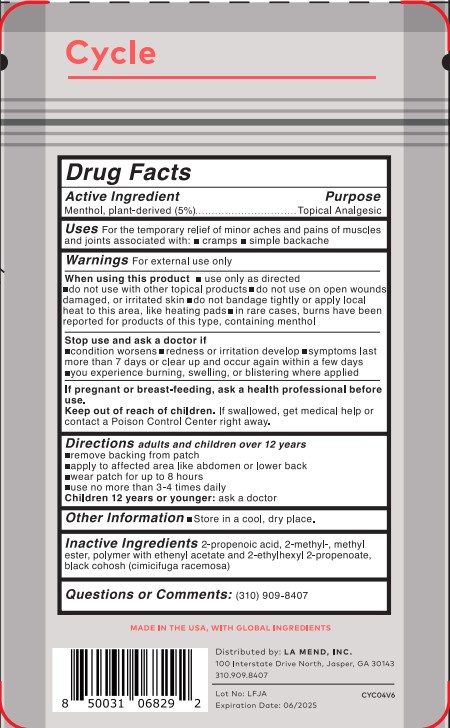
- Active Ingredient(s)
- Purpose
- Use
-
Warnings
For external use only.
When using this product
- use only as directed
- do not use with other topical products
- do not use on open wounds, damaged or irritated skin
- do not bandage tightly or apply local heat to this area, like heating pads
- in rare cases, burns have been reported for products of this type, containing menthol
- Directions
- Other information
- Inactive ingredients
- Package Label - Principal Display Panel
-
INGREDIENTS AND APPEARANCE
CYCLE
menthol patchProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:72587-001 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MENTHOL (UNII: L7T10EIP3A) (MENTHOL - UNII:L7T10EIP3A) MENTHOL 5 g in 100 g Inactive Ingredients Ingredient Name Strength 1,1'-((1-METHYL-1,2-ETHANEDIYL)2-PROPENOIC ACID, BIS(OXY(1-METHYL-2,1-ETHANEDIYL))) ESTER (UNII: 2O70KL79K2) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:72587-001-02 1 in 1 POUCH 12/06/2021 1 1.44 g in 1 PATCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M017 12/06/2021 Labeler - La Mend, Inc. (117940830)