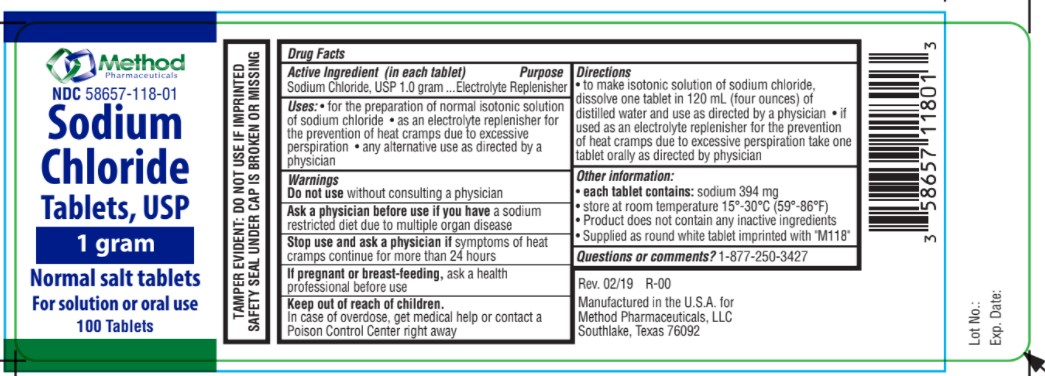
Label: SODIUM CHLORIDE NORMAL SALT- sodium chloride tablet
- NDC Code(s): 58657-118-01
- Packager: Method Pharmaceuticals
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated December 10, 2023
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Uses
- Indication
- Ask a physician
- Stop use
- If pregnant or breastfeeding,
- Keep out of reach of children
- Directions
- Warnings
- Active ingredients
- Inactive Ingredient
- Sodium Chloride
-
INGREDIENTS AND APPEARANCE
SODIUM CHLORIDE NORMAL SALT
sodium chloride tabletProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:58657-118 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SODIUM CHLORIDE (UNII: 451W47IQ8X) (CHLORIDE ION - UNII:Q32ZN48698) SODIUM CHLORIDE 1 g Product Characteristics Color white Score no score Shape ROUND Size 12mm Flavor Imprint Code M118 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:58657-118-01 100 in 1 BOTTLE; Type 0: Not a Combination Product 09/28/2021 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M018 09/23/2021 Labeler - Method Pharmaceuticals (060216698)