Label: SENNA SYRUP- natural vegetable laxative liquid
- NDC Code(s): 58657-518-08
- Packager: Method Pharmaceuticals
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 12, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
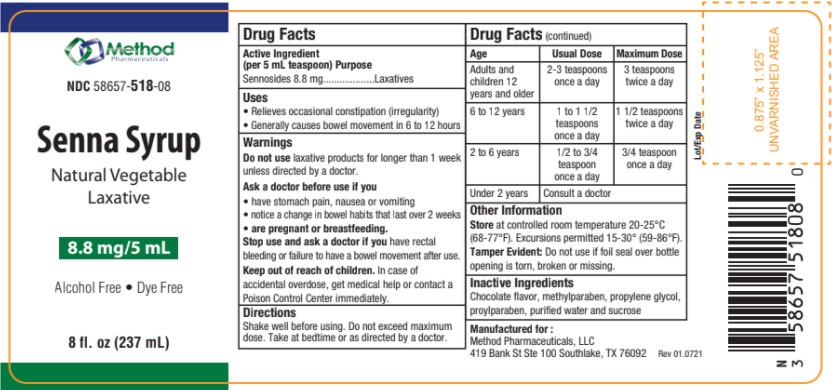
- Active Ingredients
- Purpose
- Warning
- Uses
- Ask a doctor before use if you
- Stop Use and ask a doctor
- Keep out of Reach of Children
- Directions
- OTHER SAFETY INFORMATION
- Inactive Ingredients
- Dosage
- Senna Syrup
-
INGREDIENTS AND APPEARANCE
SENNA SYRUP
natural vegetable laxative liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:58657-518 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SENNOSIDES (UNII: 3FYP5M0IJX) (SENNOSIDES - UNII:3FYP5M0IJX) SENNOSIDES 8.8 mg in 5 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) SUCROSE (UNII: C151H8M554) METHYLPARABEN (UNII: A2I8C7HI9T) PROPYLPARABEN (UNII: Z8IX2SC1OH) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:58657-518-08 237 mL in 1 BOTTLE; Type 0: Not a Combination Product 09/28/2021 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M007 09/28/2021 Labeler - Method Pharmaceuticals (060216698)