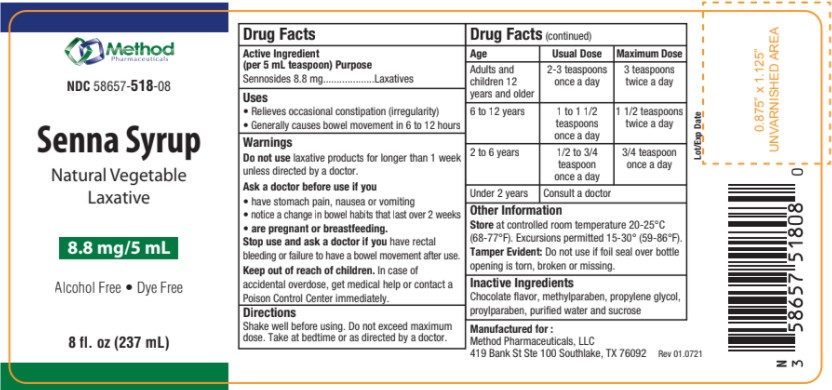
Uses
• Relieves occasional constipation (irregularity)
• Generally causes bowel movement in 6 to 12 hours
Ask a doctor before use if you
• have stomach pain, nausea or vomiting
• notice a change in bowel habits that last over 2 weeks
• are
pregnant or breastfeeding.
Stop Use and ask a doctor
if you have rectal bleeding or failure to have a bowel movement after use.
Keep out of Reach of Children
In case of accidental overdose, get medical help or contact a Poison Control Center immediately.
Directions
Shake well before using. Do not exceed maximum dose. Take at bedtime or as directed by a doctor.
Store at controlled room temperature 20-25°C(68-77°F). Excursions permitted 15-30° (59-86°F).
Tamper Evident: Do not use if foil seal over bottle opening is torn, broken or missing.
Inactive Ingredients
Chocolate flavor, methylparaben, propylene glycol, proylparaben, purified water and sucrose