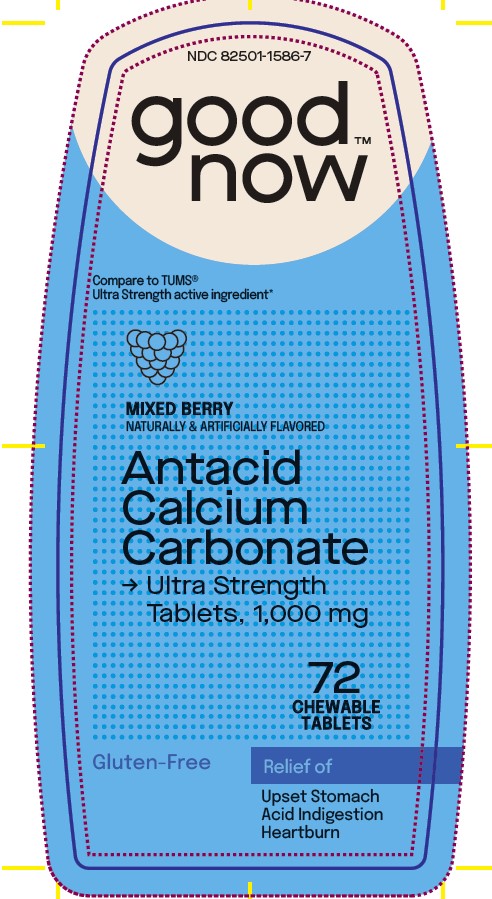
Label: ANTACID CALCIUM CARBONATE- calcium carbonate tablet, chewable
- NDC Code(s): 82501-1586-7
- Packager: Gobrands, Inc
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 8, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Active Ingredient (in each tablet)
- Purpose
- Uses
- Warnings
- Ask a doctor or pharmacist before use if you are
- When using this product
- KEEP OUT OF REACH OF CHILDREN
- Directions
- Other information
- Inactive ingredients
- Principal Display Panel
-
INGREDIENTS AND APPEARANCE
ANTACID CALCIUM CARBONATE
calcium carbonate tablet, chewableProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:82501-1586 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CALCIUM CARBONATE (UNII: H0G9379FGK) (CALCIUM CATION - UNII:2M83C4R6ZB) CALCIUM CATION 1000 mg Inactive Ingredients Ingredient Name Strength ADIPIC ACID (UNII: 76A0JE0FKJ) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) FD&C RED NO. 40 (UNII: WZB9127XOA) MAGNESIUM STEARATE (UNII: 70097M6I30) MALTODEXTRIN (UNII: 7CVR7L4A2D) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) SUCROSE (UNII: C151H8M554) Product Characteristics Color pink (Mauva, Violet) Score no score Shape ROUND Size 19mm Flavor STRAWBERRY (Raspberry, Mixed Berry) Imprint Code F16 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:82501-1586-7 72 in 1 BOTTLE; Type 0: Not a Combination Product 11/07/2023 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M001 11/07/2023 Labeler - Gobrands, Inc (057499049)