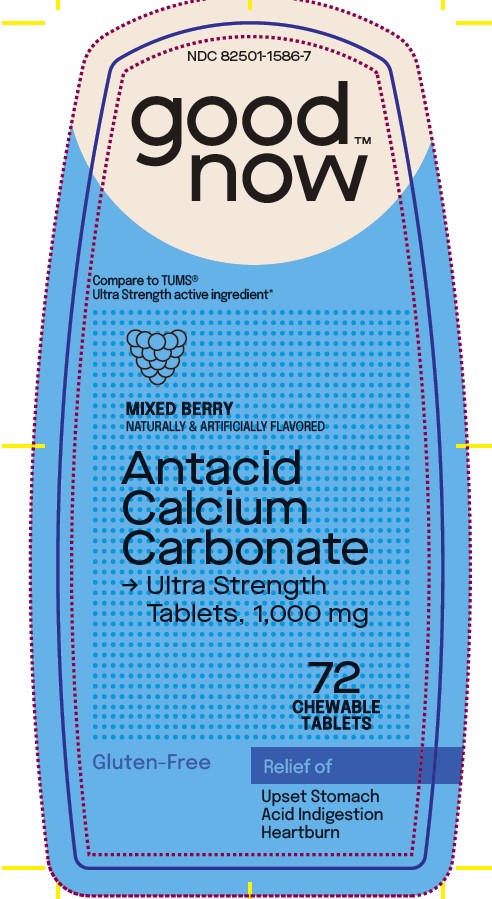
Uses
relieves
• heartburn
• acid indigestion
• sour stomach
• upsetstomach associated with these symptoms
Ask a doctor or pharmacist before use if you
are
presently taking a prescription drug. Antacids
may interact with certain prescription drugs.
When using this product
• do not take more than 7 tablets in 24 hours
• If pregnant do not take more than 5 tablets in 24 hours
• do not use the maximum dosage for more than 2 weeks except under the advice and supervision of a doctor
Directions
adults and children 12 years of age and over: chew 2 tablets to 3 tablets as symptoms occur, or as directed by a doctor
• do not take for symptoms that persist for more than 2 weeks unless advised by a doctor
Other information
• each tablet contains: elemental calcium 400 mg
• store between 20-25° C (68-77° F)