Label: BLACK LEATHER- aluminum chlorohydrate liquid
RED LEATHER- aluminum chlorohydrate liquid
BROWN LEATHER- aluminum chlorohydrate liquid
GREEN LEATHER- aluminum chlorohydrate liquid
ORANGE LEATHER- aluminum chlorohydrate liquid
PRESIDENTIAL LEATHER- aluminum chlorohydrate liquid
SILVER LEATHER- aluminum chlorohydrate liquid
BLUE LEATHER- aluminum chlorohydrate liquid
PURPLE LEATHER- aluminum chlorohydrate liquid
FUTURE TIME- aluminum chlorohydrate liquid
PRECIOUS GEMS- aluminum chlorohydrate liquid
SEXY TASHA- aluminum chlorohydrate liquid
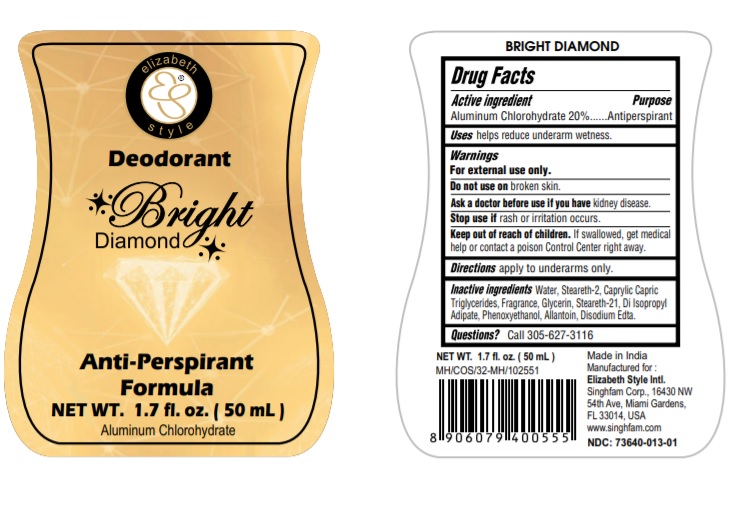
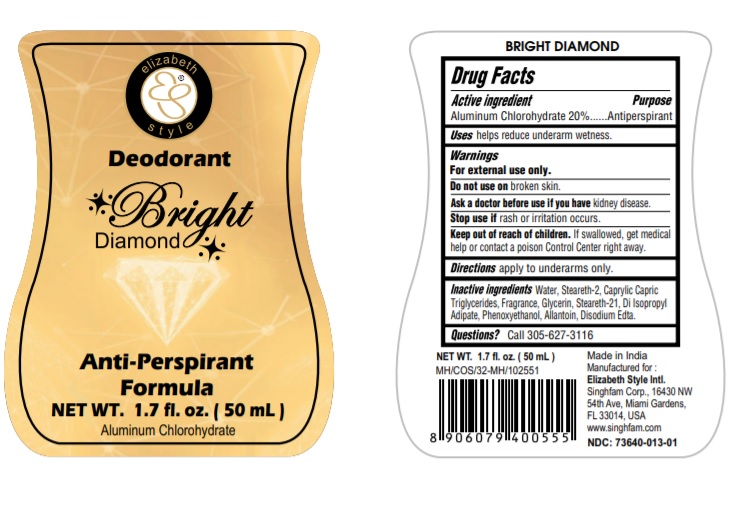
BRIGHT DIAMOND- aluminum chlorohydrate liquid
RED ROSE- aluminum chlorohydrate liquid
BE DAZZLE- aluminum chlorohydrate liquid
AFRICAN OPIUM- aluminum chlorohydrate liquid
PINK SATIN LACE- aluminum chlorohydrate liquid
SWEET ONES- aluminum chlorohydrate liquid
-
Contains inactivated NDC Code(s)
NDC Code(s): 73640-001-01, 73640-002-01, 73640-003-01, 73640-004-01, view more73640-005-01, 73640-006-01, 73640-007-01, 73640-008-01, 73640-009-01, 73640-010-01, 73640-011-01, 73640-012-01, 73640-013-01, 73640-014-01, 73640-015-01, 73640-016-01, 73640-017-01, 73640-018-01 - Packager: N. N. IMPEX
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated May 6, 2021
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredients
- Purpose
- Warnings
- Keep out of reach of children.
- Use
- Directions
- SPL UNCLASSIFIED SECTION
- Inactive Ingredients
- Product label
- Product label
- Product label
- Product label
- Product label
- Product label
- Product label
- Product label
- Product label
- Product label
- Product label
- Product label
- Product label
- Product label
- Product label
- Product label
- Product label
- Product label
-
INGREDIENTS AND APPEARANCE
BLACK LEATHER
aluminum chlorohydrate liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:73640-003 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALUMINUM CHLOROHYDRATE (UNII: HPN8MZW13M) (ALUMINUM CHLOROHYDRATE - UNII:HPN8MZW13M) ALUMINUM CHLOROHYDRATE 200 mg in 1 mL Inactive Ingredients Ingredient Name Strength STEARETH-2 (UNII: V56DFE46J5) STEARETH-21 (UNII: 53J3F32P58) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) DIISOPROPYL ADIPATE (UNII: P7E6YFV72X) EDETATE DISODIUM (UNII: 7FLD91C86K) GLYCERIN (UNII: PDC6A3C0OX) ALLANTOIN (UNII: 344S277G0Z) PHENOXYETHANOL (UNII: HIE492ZZ3T) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:73640-003-01 50 mL in 1 BOTTLE; Type 0: Not a Combination Product 05/27/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part350 05/27/2020 RED LEATHER
aluminum chlorohydrate liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:73640-001 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALUMINUM CHLOROHYDRATE (UNII: HPN8MZW13M) (ALUMINUM CHLOROHYDRATE - UNII:HPN8MZW13M) ALUMINUM CHLOROHYDRATE 200 mg in 1 mL Inactive Ingredients Ingredient Name Strength STEARETH-2 (UNII: V56DFE46J5) STEARETH-21 (UNII: 53J3F32P58) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) DIISOPROPYL ADIPATE (UNII: P7E6YFV72X) EDETATE DISODIUM (UNII: 7FLD91C86K) GLYCERIN (UNII: PDC6A3C0OX) ALLANTOIN (UNII: 344S277G0Z) PHENOXYETHANOL (UNII: HIE492ZZ3T) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:73640-001-01 50 mL in 1 BOTTLE; Type 0: Not a Combination Product 05/27/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part350 05/27/2020 BROWN LEATHER
aluminum chlorohydrate liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:73640-002 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALUMINUM CHLOROHYDRATE (UNII: HPN8MZW13M) (ALUMINUM CHLOROHYDRATE - UNII:HPN8MZW13M) ALUMINUM CHLOROHYDRATE 200 mg in 1 mL Inactive Ingredients Ingredient Name Strength STEARETH-2 (UNII: V56DFE46J5) STEARETH-21 (UNII: 53J3F32P58) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) DIISOPROPYL ADIPATE (UNII: P7E6YFV72X) EDETATE DISODIUM (UNII: 7FLD91C86K) GLYCERIN (UNII: PDC6A3C0OX) ALLANTOIN (UNII: 344S277G0Z) PHENOXYETHANOL (UNII: HIE492ZZ3T) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:73640-002-01 50 mL in 1 BOTTLE; Type 0: Not a Combination Product 05/28/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part350 05/28/2020 GREEN LEATHER
aluminum chlorohydrate liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:73640-004 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALUMINUM CHLOROHYDRATE (UNII: HPN8MZW13M) (ALUMINUM CHLOROHYDRATE - UNII:HPN8MZW13M) ALUMINUM CHLOROHYDRATE 200 mg in 1 mL Inactive Ingredients Ingredient Name Strength STEARETH-2 (UNII: V56DFE46J5) STEARETH-21 (UNII: 53J3F32P58) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) DIISOPROPYL ADIPATE (UNII: P7E6YFV72X) EDETATE DISODIUM (UNII: 7FLD91C86K) GLYCERIN (UNII: PDC6A3C0OX) ALLANTOIN (UNII: 344S277G0Z) PHENOXYETHANOL (UNII: HIE492ZZ3T) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:73640-004-01 50 mL in 1 BOTTLE; Type 0: Not a Combination Product 05/28/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part350 05/28/2020 ORANGE LEATHER
aluminum chlorohydrate liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:73640-005 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALUMINUM CHLOROHYDRATE (UNII: HPN8MZW13M) (ALUMINUM CHLOROHYDRATE - UNII:HPN8MZW13M) ALUMINUM CHLOROHYDRATE 200 mg in 1 mL Inactive Ingredients Ingredient Name Strength STEARETH-2 (UNII: V56DFE46J5) STEARETH-21 (UNII: 53J3F32P58) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) DIISOPROPYL ADIPATE (UNII: P7E6YFV72X) EDETATE DISODIUM (UNII: 7FLD91C86K) GLYCERIN (UNII: PDC6A3C0OX) ALLANTOIN (UNII: 344S277G0Z) PHENOXYETHANOL (UNII: HIE492ZZ3T) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:73640-005-01 50 mL in 1 BOTTLE; Type 0: Not a Combination Product 05/28/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part350 05/28/2020 PRESIDENTIAL LEATHER
aluminum chlorohydrate liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:73640-006 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALUMINUM CHLOROHYDRATE (UNII: HPN8MZW13M) (ALUMINUM CHLOROHYDRATE - UNII:HPN8MZW13M) ALUMINUM CHLOROHYDRATE 200 mg in 1 mL Inactive Ingredients Ingredient Name Strength STEARETH-2 (UNII: V56DFE46J5) STEARETH-21 (UNII: 53J3F32P58) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) DIISOPROPYL ADIPATE (UNII: P7E6YFV72X) EDETATE DISODIUM (UNII: 7FLD91C86K) GLYCERIN (UNII: PDC6A3C0OX) ALLANTOIN (UNII: 344S277G0Z) PHENOXYETHANOL (UNII: HIE492ZZ3T) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:73640-006-01 50 mL in 1 BOTTLE; Type 0: Not a Combination Product 05/28/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part350 05/28/2020 SILVER LEATHER
aluminum chlorohydrate liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:73640-007 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALUMINUM CHLOROHYDRATE (UNII: HPN8MZW13M) (ALUMINUM CHLOROHYDRATE - UNII:HPN8MZW13M) ALUMINUM CHLOROHYDRATE 200 mg in 1 mL Inactive Ingredients Ingredient Name Strength STEARETH-2 (UNII: V56DFE46J5) STEARETH-21 (UNII: 53J3F32P58) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) DIISOPROPYL ADIPATE (UNII: P7E6YFV72X) EDETATE DISODIUM (UNII: 7FLD91C86K) GLYCERIN (UNII: PDC6A3C0OX) ALLANTOIN (UNII: 344S277G0Z) PHENOXYETHANOL (UNII: HIE492ZZ3T) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:73640-007-01 50 mL in 1 BOTTLE; Type 0: Not a Combination Product 05/28/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part350 05/28/2020 BLUE LEATHER
aluminum chlorohydrate liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:73640-008 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALUMINUM CHLOROHYDRATE (UNII: HPN8MZW13M) (ALUMINUM CHLOROHYDRATE - UNII:HPN8MZW13M) ALUMINUM CHLOROHYDRATE 200 mg in 1 mL Inactive Ingredients Ingredient Name Strength STEARETH-2 (UNII: V56DFE46J5) STEARETH-21 (UNII: 53J3F32P58) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) DIISOPROPYL ADIPATE (UNII: P7E6YFV72X) EDETATE DISODIUM (UNII: 7FLD91C86K) GLYCERIN (UNII: PDC6A3C0OX) ALLANTOIN (UNII: 344S277G0Z) PHENOXYETHANOL (UNII: HIE492ZZ3T) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:73640-008-01 50 mL in 1 BOTTLE; Type 0: Not a Combination Product 05/28/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part350 05/28/2020 PURPLE LEATHER
aluminum chlorohydrate liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:73640-009 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALUMINUM CHLOROHYDRATE (UNII: HPN8MZW13M) (ALUMINUM CHLOROHYDRATE - UNII:HPN8MZW13M) ALUMINUM CHLOROHYDRATE 200 mg in 1 mL Inactive Ingredients Ingredient Name Strength STEARETH-2 (UNII: V56DFE46J5) STEARETH-21 (UNII: 53J3F32P58) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) DIISOPROPYL ADIPATE (UNII: P7E6YFV72X) EDETATE DISODIUM (UNII: 7FLD91C86K) GLYCERIN (UNII: PDC6A3C0OX) ALLANTOIN (UNII: 344S277G0Z) PHENOXYETHANOL (UNII: HIE492ZZ3T) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:73640-009-01 50 mL in 1 BOTTLE; Type 0: Not a Combination Product 05/28/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part350 05/28/2020 FUTURE TIME
aluminum chlorohydrate liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:73640-010 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALUMINUM CHLOROHYDRATE (UNII: HPN8MZW13M) (ALUMINUM CHLOROHYDRATE - UNII:HPN8MZW13M) ALUMINUM CHLOROHYDRATE 200 mg in 1 mL Inactive Ingredients Ingredient Name Strength STEARETH-2 (UNII: V56DFE46J5) STEARETH-21 (UNII: 53J3F32P58) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) DIISOPROPYL ADIPATE (UNII: P7E6YFV72X) EDETATE DISODIUM (UNII: 7FLD91C86K) GLYCERIN (UNII: PDC6A3C0OX) ALLANTOIN (UNII: 344S277G0Z) PHENOXYETHANOL (UNII: HIE492ZZ3T) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:73640-010-01 50 mL in 1 BOTTLE; Type 0: Not a Combination Product 05/28/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part350 05/28/2020 PRECIOUS GEMS
aluminum chlorohydrate liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:73640-011 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALUMINUM CHLOROHYDRATE (UNII: HPN8MZW13M) (ALUMINUM CHLOROHYDRATE - UNII:HPN8MZW13M) ALUMINUM CHLOROHYDRATE 200 mg in 1 mL Inactive Ingredients Ingredient Name Strength STEARETH-2 (UNII: V56DFE46J5) STEARETH-21 (UNII: 53J3F32P58) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) DIISOPROPYL ADIPATE (UNII: P7E6YFV72X) EDETATE DISODIUM (UNII: 7FLD91C86K) GLYCERIN (UNII: PDC6A3C0OX) ALLANTOIN (UNII: 344S277G0Z) PHENOXYETHANOL (UNII: HIE492ZZ3T) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:73640-011-01 50 mL in 1 BOTTLE; Type 0: Not a Combination Product 05/28/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part350 05/28/2020 SEXY TASHA
aluminum chlorohydrate liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:73640-012 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALUMINUM CHLOROHYDRATE (UNII: HPN8MZW13M) (ALUMINUM CHLOROHYDRATE - UNII:HPN8MZW13M) ALUMINUM CHLOROHYDRATE 200 mg in 1 mL Inactive Ingredients Ingredient Name Strength STEARETH-2 (UNII: V56DFE46J5) STEARETH-21 (UNII: 53J3F32P58) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) DIISOPROPYL ADIPATE (UNII: P7E6YFV72X) EDETATE DISODIUM (UNII: 7FLD91C86K) GLYCERIN (UNII: PDC6A3C0OX) ALLANTOIN (UNII: 344S277G0Z) PHENOXYETHANOL (UNII: HIE492ZZ3T) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:73640-012-01 50 mL in 1 BOTTLE; Type 0: Not a Combination Product 05/28/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part350 05/28/2020 BRIGHT DIAMOND
aluminum chlorohydrate liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:73640-013 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALUMINUM CHLOROHYDRATE (UNII: HPN8MZW13M) (ALUMINUM CHLOROHYDRATE - UNII:HPN8MZW13M) ALUMINUM CHLOROHYDRATE 200 mg in 1 mL Inactive Ingredients Ingredient Name Strength STEARETH-2 (UNII: V56DFE46J5) STEARETH-21 (UNII: 53J3F32P58) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) DIISOPROPYL ADIPATE (UNII: P7E6YFV72X) EDETATE DISODIUM (UNII: 7FLD91C86K) GLYCERIN (UNII: PDC6A3C0OX) ALLANTOIN (UNII: 344S277G0Z) PHENOXYETHANOL (UNII: HIE492ZZ3T) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:73640-013-01 50 mL in 1 BOTTLE; Type 0: Not a Combination Product 05/28/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part350 05/28/2020 RED ROSE
aluminum chlorohydrate liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:73640-014 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALUMINUM CHLOROHYDRATE (UNII: HPN8MZW13M) (ALUMINUM CHLOROHYDRATE - UNII:HPN8MZW13M) ALUMINUM CHLOROHYDRATE 200 mg in 1 mL Inactive Ingredients Ingredient Name Strength STEARETH-2 (UNII: V56DFE46J5) STEARETH-21 (UNII: 53J3F32P58) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) DIISOPROPYL ADIPATE (UNII: P7E6YFV72X) EDETATE DISODIUM (UNII: 7FLD91C86K) GLYCERIN (UNII: PDC6A3C0OX) ALLANTOIN (UNII: 344S277G0Z) PHENOXYETHANOL (UNII: HIE492ZZ3T) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:73640-014-01 50 mL in 1 BOTTLE; Type 0: Not a Combination Product 05/28/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part350 05/28/2020 BE DAZZLE
aluminum chlorohydrate liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:73640-015 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALUMINUM CHLOROHYDRATE (UNII: HPN8MZW13M) (ALUMINUM CHLOROHYDRATE - UNII:HPN8MZW13M) ALUMINUM CHLOROHYDRATE 200 mg in 1 mL Inactive Ingredients Ingredient Name Strength STEARETH-2 (UNII: V56DFE46J5) STEARETH-21 (UNII: 53J3F32P58) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) DIISOPROPYL ADIPATE (UNII: P7E6YFV72X) EDETATE DISODIUM (UNII: 7FLD91C86K) GLYCERIN (UNII: PDC6A3C0OX) ALLANTOIN (UNII: 344S277G0Z) PHENOXYETHANOL (UNII: HIE492ZZ3T) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:73640-015-01 50 mL in 1 BOTTLE; Type 0: Not a Combination Product 05/28/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part350 05/28/2020 AFRICAN OPIUM
aluminum chlorohydrate liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:73640-016 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALUMINUM CHLOROHYDRATE (UNII: HPN8MZW13M) (ALUMINUM CHLOROHYDRATE - UNII:HPN8MZW13M) ALUMINUM CHLOROHYDRATE 200 mg in 1 mL Inactive Ingredients Ingredient Name Strength STEARETH-2 (UNII: V56DFE46J5) STEARETH-21 (UNII: 53J3F32P58) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) DIISOPROPYL ADIPATE (UNII: P7E6YFV72X) EDETATE DISODIUM (UNII: 7FLD91C86K) GLYCERIN (UNII: PDC6A3C0OX) ALLANTOIN (UNII: 344S277G0Z) PHENOXYETHANOL (UNII: HIE492ZZ3T) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:73640-016-01 50 mL in 1 BOTTLE; Type 0: Not a Combination Product 05/28/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part350 05/28/2020 PINK SATIN LACE
aluminum chlorohydrate liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:73640-017 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALUMINUM CHLOROHYDRATE (UNII: HPN8MZW13M) (ALUMINUM CHLOROHYDRATE - UNII:HPN8MZW13M) ALUMINUM CHLOROHYDRATE 200 mg in 1 mL Inactive Ingredients Ingredient Name Strength STEARETH-2 (UNII: V56DFE46J5) STEARETH-21 (UNII: 53J3F32P58) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) DIISOPROPYL ADIPATE (UNII: P7E6YFV72X) EDETATE DISODIUM (UNII: 7FLD91C86K) GLYCERIN (UNII: PDC6A3C0OX) ALLANTOIN (UNII: 344S277G0Z) PHENOXYETHANOL (UNII: HIE492ZZ3T) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:73640-017-01 50 mL in 1 BOTTLE; Type 0: Not a Combination Product 05/28/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part350 05/28/2020 SWEET ONES
aluminum chlorohydrate liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:73640-018 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALUMINUM CHLOROHYDRATE (UNII: HPN8MZW13M) (ALUMINUM CHLOROHYDRATE - UNII:HPN8MZW13M) ALUMINUM CHLOROHYDRATE 200 mg in 1 mL Inactive Ingredients Ingredient Name Strength STEARETH-2 (UNII: V56DFE46J5) STEARETH-21 (UNII: 53J3F32P58) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) DIISOPROPYL ADIPATE (UNII: P7E6YFV72X) EDETATE DISODIUM (UNII: 7FLD91C86K) GLYCERIN (UNII: PDC6A3C0OX) ALLANTOIN (UNII: 344S277G0Z) PHENOXYETHANOL (UNII: HIE492ZZ3T) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:73640-018-01 50 mL in 1 BOTTLE; Type 0: Not a Combination Product 05/28/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part350 05/28/2020 Labeler - N. N. IMPEX (915969592)