BLACK LEATHER- aluminum chlorohydrate liquid
RED LEATHER- aluminum chlorohydrate liquid
BROWN LEATHER- aluminum chlorohydrate liquid
GREEN LEATHER- aluminum chlorohydrate liquid
ORANGE LEATHER- aluminum chlorohydrate liquid
PRESIDENTIAL LEATHER- aluminum chlorohydrate liquid
SILVER LEATHER- aluminum chlorohydrate liquid
BLUE LEATHER- aluminum chlorohydrate liquid
PURPLE LEATHER- aluminum chlorohydrate liquid
FUTURE TIME- aluminum chlorohydrate liquid
PRECIOUS GEMS- aluminum chlorohydrate liquid
SEXY TASHA- aluminum chlorohydrate liquid
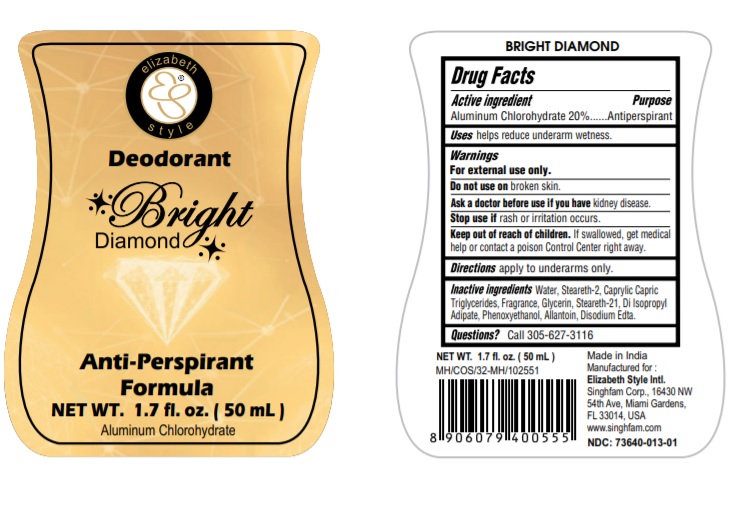
BRIGHT DIAMOND- aluminum chlorohydrate liquid
RED ROSE- aluminum chlorohydrate liquid
BE DAZZLE- aluminum chlorohydrate liquid
AFRICAN OPIUM- aluminum chlorohydrate liquid
PINK SATIN LACE- aluminum chlorohydrate liquid
SWEET ONES- aluminum chlorohydrate liquid
N. N. IMPEX
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Active ingredients
Aluminum Chlorohydrate 20%
Warnings
Warnings For external use only.
Do not use on broken skin.
Ask a doctor before use if you have kidney disease.
Stop use if rash or irritation occurs
Keep out of reach of children.
If swallowed, get medical help or contact a poison Control Center right away
Use
helps reduce underarm wetness
Directions
apply to underarms only.
Questions? Call 305-627-3116
Inactive Ingredients
Water,Steareth-2, Caprylic Capric
Triglycerides, Fragrance, Glycerin, Steareth-21, Di Isopropyl
Adipate, Phenoxyethanol, Allantoin, Disodium Edta
Product label

Product label

Product label

Product label

Product label

Product label

Product label

Product label

Product label

Product label

Product label

Product label

Product label
Product label

Product label

Product label

Product label

Product label

N. N. IMPEX

















