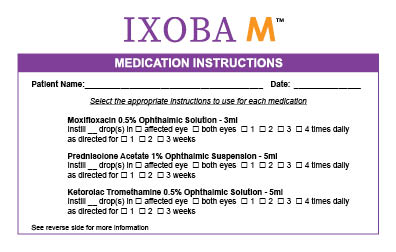
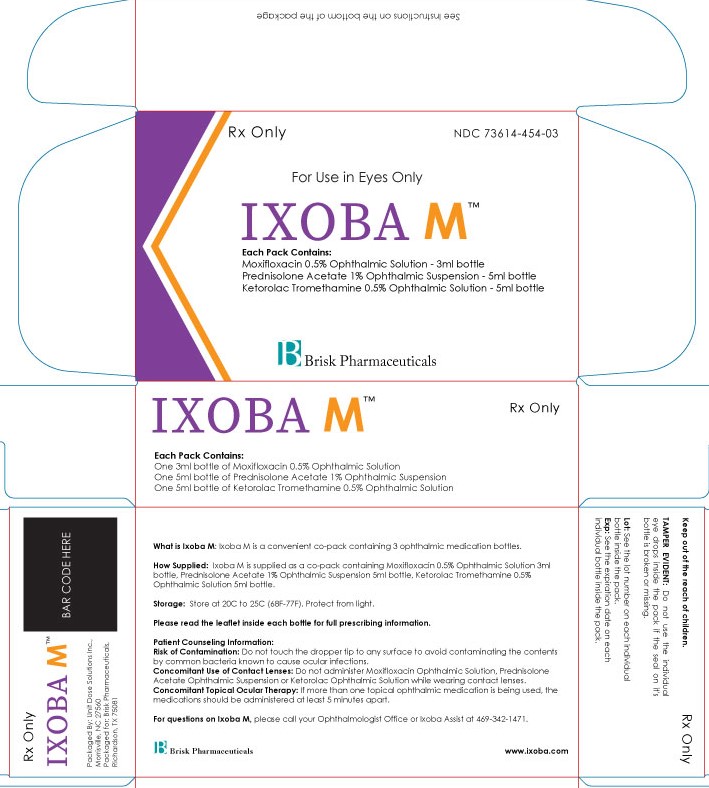
Label: IXOBA M- moxifloxacin 0.5%, ketorolac 0.5%, prednisolone acetate 1% kit
-
Contains inactivated NDC Code(s)
NDC Code(s): 73614-454-03 - Packager: Brisk Pharmaceuticals, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated November 3, 2021
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- DESCRIPTION
-
DESCRIPTION
See Instructions on the bottom of the package
What is Ixoba M used for: Ixoba M is a convenient pack containing 3 ophthalmic medication bottles.
How Supplied: Ixoba M is supplied as a co-pack containing Moxifloxacin 0.5% Ophthalmic Suspension 3ml bottle, Ketorolac 0.5% Ophthalmic Suspension 5ml bottle, Prednisolone Acetate 1% Ophthalmic Suspen-sion 5ml bottle.
Storage: Store at 20C to 25C (68F-77F). Protect from light.
Please read the leaflet inside each bottle for ‘full prescribing information’ about that medication.
Patient Counseling Information:
Risk of Contamination: Do not touch the dropper tip to any surface to avoid contaminating the contents by common bacteria known to cause ocular infections.
Concomitant Use of Contact Lenses: Do not administer Moxifloxacin Ophthalmic Suspension, Ketorolac Ophthalmic Suspension or Prednisolone Acetate Ophthalmic Suspension while wearing contact lenses.
Concomitant Topical Ocular Therapy: If more than one topical ophthalmic medication is being used, the medications should be administered at least 5 minutes apart.
For questions on Ixoba M, please call your Ophthalmologist Office or Ixoba Assist at 469-342-1471.
-
DESCRIPTION
Keep out of the reach of children.
TAMPER EVIDENT: Do not use the individual eye drops inside the pack if the seal on its carton is broken or missing.
Lot: See the lot number on each individual bottle inside the pack.
Exp: See the expiration date on each individual bottle inside the pack.
Packaged By: Unit Dose Solutions Inc., Morrisville, NC 27560
Packaged for: Brisk Pharmaceuticals, Dallas, TX 75217
- PATIENT MEDICATION INFORMATION
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
IXOBA M
moxifloxacin 0.5%, ketorolac 0.5%, prednisolone acetate 1% kitProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:73614-454 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:73614-454-03 1 in 1 CARTON; Type 1: Convenience Kit of Co-Package 08/26/2021 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 BOTTLE 5 mL Part 2 1 BOTTLE 5 mL Part 3 1 BOTTLE 3 mL Part 1 of 3 KETOROLAC TROMETHAMINE
ketorolac tromethamine solutionProduct Information Item Code (Source) NDC:61314-126 Route of Administration OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength KETOROLAC TROMETHAMINE (UNII: 4EVE5946BQ) (KETOROLAC - UNII:YZI5105V0L) KETOROLAC 5 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) EDETATE DISODIUM (UNII: 7FLD91C86K) SODIUM CHLORIDE (UNII: 451W47IQ8X) HYDROCHLORIC ACID (UNII: QTT17582CB) BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) SODIUM HYDROXIDE (UNII: 55X04QC32I) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 5 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA076583 11/05/2009 Part 2 of 3 PREDNISOLONE ACETATE
prednisolone acetate suspension/ dropsProduct Information Item Code (Source) NDC:60758-119 Route of Administration OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PREDNISOLONE ACETATE (UNII: 8B2807733D) (PREDNISOLONE - UNII:9PHQ9Y1OLM) PREDNISOLONE ACETATE 10 mg in 1 mL Inactive Ingredients Ingredient Name Strength POLYSORBATE 80 (UNII: 6OZP39ZG8H) SODIUM CITRATE (UNII: 1Q73Q2JULR) EDETATE DISODIUM (UNII: 7FLD91C86K) WATER (UNII: 059QF0KO0R) SODIUM BISULFITE (UNII: TZX5469Z6I) SODIUM CHLORIDE (UNII: 451W47IQ8X) BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) BORIC ACID (UNII: R57ZHV85D4) HYPROMELLOSES (UNII: 3NXW29V3WO) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 1 in 1 CARTON 1 5 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA authorized generic NDA017011 08/19/1997 Part 3 of 3 MOXIFLOXACIN
moxifloxacin solution/ dropsProduct Information Item Code (Source) NDC:68180-422 Route of Administration OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MOXIFLOXACIN HYDROCHLORIDE MONOHYDRATE (UNII: B8956S8609) (MOXIFLOXACIN - UNII:U188XYD42P) MOXIFLOXACIN 5 mg in 1 mL Inactive Ingredients Ingredient Name Strength BORIC ACID (UNII: R57ZHV85D4) HYDROCHLORIC ACID (UNII: QTT17582CB) SODIUM CHLORIDE (UNII: 451W47IQ8X) SODIUM HYDROXIDE (UNII: 55X04QC32I) WATER (UNII: 059QF0KO0R) Product Characteristics Color yellow (Yellow Colored Transparent) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 1 in 1 CARTON 1 3 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA202867 07/01/2017 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 08/26/2021 Labeler - Brisk Pharmaceuticals, Inc. (117250794) Establishment Name Address ID/FEI Business Operations Unit Dose Solutions, Inc 360804194 repack(73614-454)