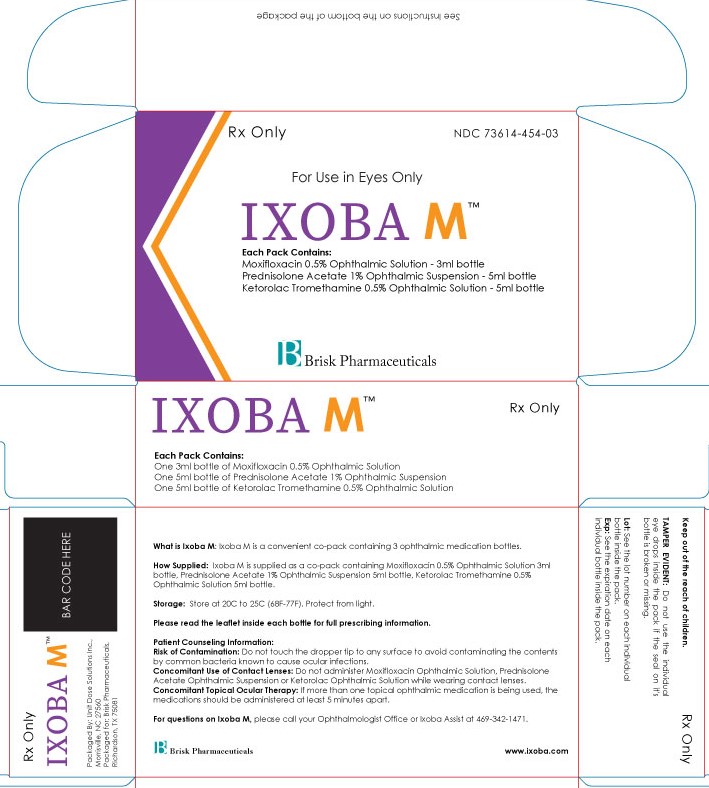
Rx Only
NDC 73614-454-03
For Use in Eyes Only
IXOBA M
Each Pack Contains:
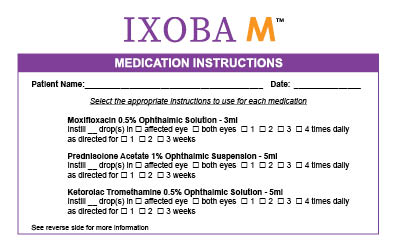
Moxifloxacin 0.5% Ophthalmic Solution - 3ml
Ketorolac 0.5% Ophthalmic Solution - 5ml
Prednisolone Acetate 1% Ophthalmic Suspension - 5ml
Brisk Pharmaceuticals
See Instructions on the bottom of the package
What is Ixoba M used for: Ixoba M is a convenient pack containing 3 ophthalmic medication bottles.
How Supplied: Ixoba M is supplied as a co-pack containing Moxifloxacin 0.5% Ophthalmic Suspension 3ml bottle, Ketorolac 0.5% Ophthalmic Suspension 5ml bottle, Prednisolone Acetate 1% Ophthalmic Suspen-sion 5ml bottle.
Storage: Store at 20C to 25C (68F-77F). Protect from light.
Please read the leaflet inside each bottle for ‘full prescribing information’ about that medication.
Patient Counseling Information:
Risk of Contamination: Do not touch the dropper tip to any surface to avoid contaminating the contents by common bacteria known to cause ocular infections.
Concomitant Use of Contact Lenses: Do not administer Moxifloxacin Ophthalmic Suspension, Ketorolac Ophthalmic Suspension or Prednisolone Acetate Ophthalmic Suspension while wearing contact lenses.
Concomitant Topical Ocular Therapy: If more than one topical ophthalmic medication is being used, the medications should be administered at least 5 minutes apart.
For questions on Ixoba M, please call your Ophthalmologist Office or Ixoba Assist at 469-342-1471.
Keep out of the reach of children.
TAMPER EVIDENT: Do not use the individual eye drops inside the pack if the seal on its carton is broken or missing.
Lot: See the lot number on each individual bottle inside the pack.
Exp: See the expiration date on each individual bottle inside the pack.
Packaged By: Unit Dose Solutions Inc., Morrisville, NC 27560
Packaged for: Brisk Pharmaceuticals, Dallas, TX 75217