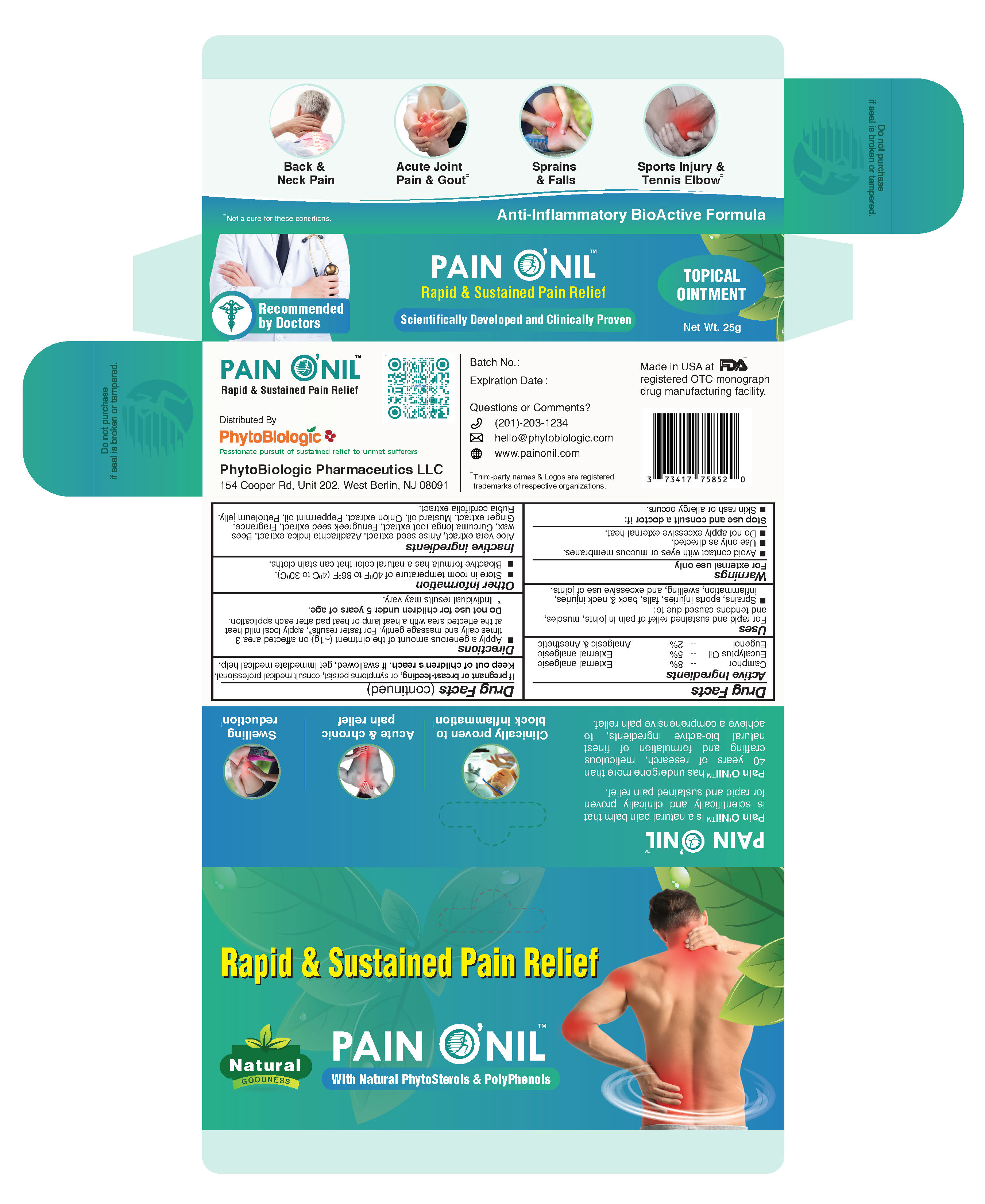
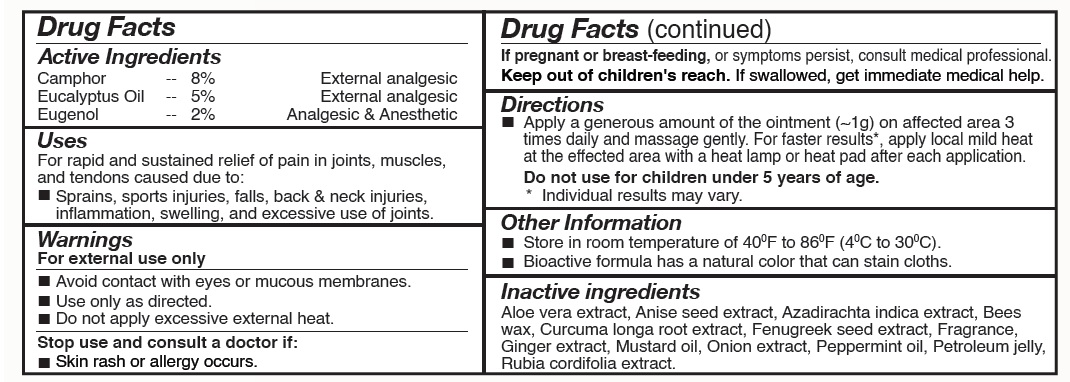
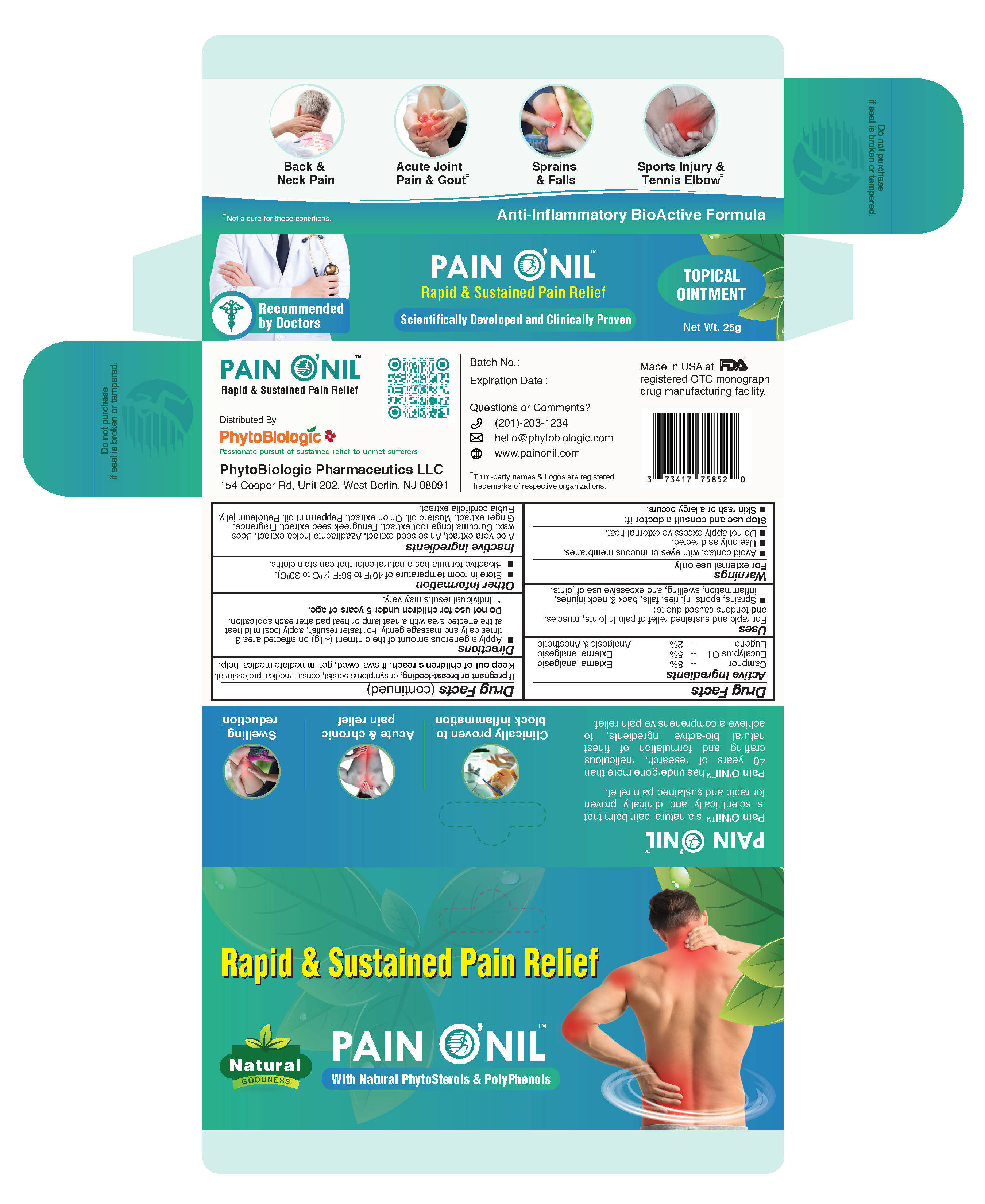
Label: PAINONIL- eugenol, camphor, eucalyptus ointment
-
Contains inactivated NDC Code(s)
NDC Code(s): 73417-758-52 - Packager: PhytoBiologic Pharmaceutics LLC
- Category: HUMAN OTC DRUG LABEL
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated July 24, 2021
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- PainONil Active Ingredients
- Purpose
- Uses
-
Warnings
- For external use only.
- Avoid contact with eyes and mucous membranes.
- Use only as directed.
- Stop use and consult a doctor if: Skin rash or allergy occurs.
- If pregnant or breast-feeding, or symptoms persist, consult medical professional.
- Keep out of children's reach. If swallowed, get immediate medical help.
- Directions
- When Using This Product
- Stop Use Section
- If pregnant or breat feeding
- Keep Out of Reach of Children
- Other Information
- Inactive Ingredients
- Questions or Comments ?
- Principal Display Panel - 25 g Tube & Carton
-
INGREDIENTS AND APPEARANCE
PAINONIL
eugenol, camphor, eucalyptus ointmentProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:73417-758 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CAMPHOR (NATURAL) (UNII: N20HL7Q941) (CAMPHOR (NATURAL) - UNII:N20HL7Q941) CAMPHOR (NATURAL) 80 mg in 1 g EUGENOL (UNII: 3T8H1794QW) (EUGENOL - UNII:3T8H1794QW) EUGENOL 20 mg in 1 g EUCALYPTUS OIL (UNII: 2R04ONI662) (EUCALYPTUS OIL - UNII:2R04ONI662) EUCALYPTUS OIL 50 mg in 1 g Inactive Ingredients Ingredient Name Strength FENUGREEK SEED (UNII: 654825W09Z) MUSTARD OIL (UNII: TYY1MA9BSY) PETROLATUM (UNII: 4T6H12BN9U) PEPPERMINT OIL (UNII: AV092KU4JH) AZADIRACHTA INDICA LEAF (UNII: HKY915780T) YELLOW WAX (UNII: 2ZA36H0S2V) RUBIA CORDIFOLIA ROOT (UNII: 4V873H15CG) ALOE VERA LEAF (UNII: ZY81Z83H0X) TURMERIC (UNII: 856YO1Z64F) ONION JUICE (UNII: 77539R6FGN) GINGER (UNII: C5529G5JPQ) ANISE OIL (UNII: 6Y89129C8H) Product Characteristics Color yellow Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:73417-758-52 1 in 1 CARTON 08/12/2021 1 25 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part348 08/12/2021 Labeler - PhytoBiologic Pharmaceutics LLC (117103844) Registrant - PhytoBiologic Pharmaceutics LLC (117103844) Establishment Name Address ID/FEI Business Operations PhytoBiologic Pharmaceutics LLC 117103844 manufacture(73417-758) , label(73417-758)