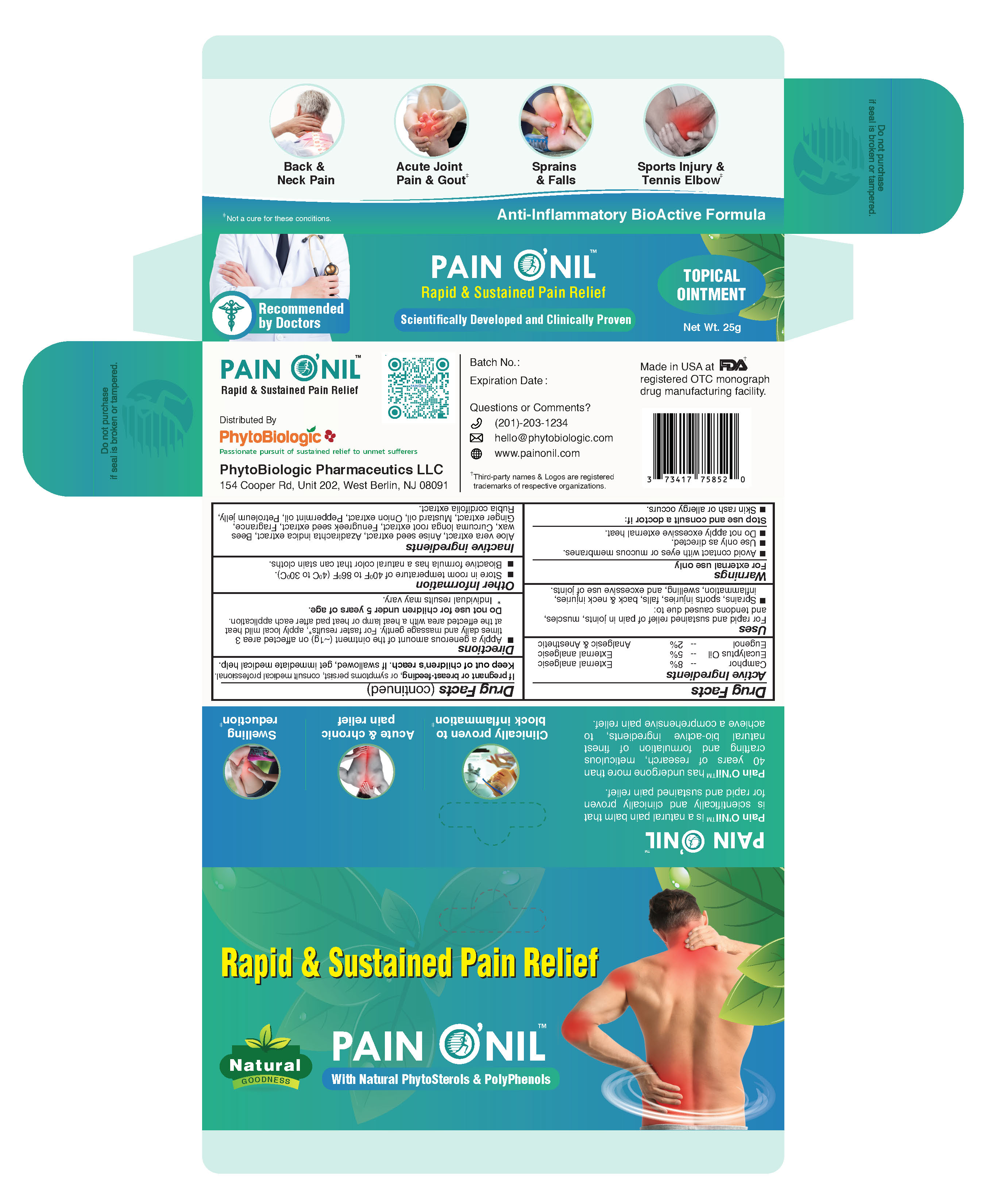
PainONil Active Ingredients
|
Active Ingredient |
Strength |
Purpose |
|
Camphor |
8% |
External analgesic |
|
Eucalyptus oil |
5% |
External analgesic |
| Eugenol | 2% | Analgesic & Anesthetic |
Uses
For rapid and sustained relief of pain in joints, muscles, and tendons caused due to:
- Sprains
- sports injuries
- falls
- back & neck injuries
- inflammation
- swelling
- excessive use of joints
Warnings
- For external use only.
- Avoid contact with eyes and mucous membranes.
- Use only as directed.
- Stop use and consult a doctor if: Skin rash or allergy occurs.
- If pregnant or breast-feeding, or symptoms persist, consult medical professional.
- Keep out of children's reach. If swallowed, get immediate medical help.
Directions
Apply a generous amount of the ointment (~1g) on the affected area 3 times daily and massage gently. For faster results*, apply mild heat at the effected area with a heat lamp or heat pad after each application.
Do not use for children under 5 years of age.
* Individual results may vary.
If pregnant or breat feeding
If pregnant or breast-feeding, or symptoms persist, consult medical professional before use
Keep Out of Reach of Children
Keep out of children's reach. If swallowed, get immediate medical help.
Other Information
- Store in room temperature 40 0F to 86 0F (4 0C to 30 0C)
- Bioactive formula has a natural color that can stain cloths