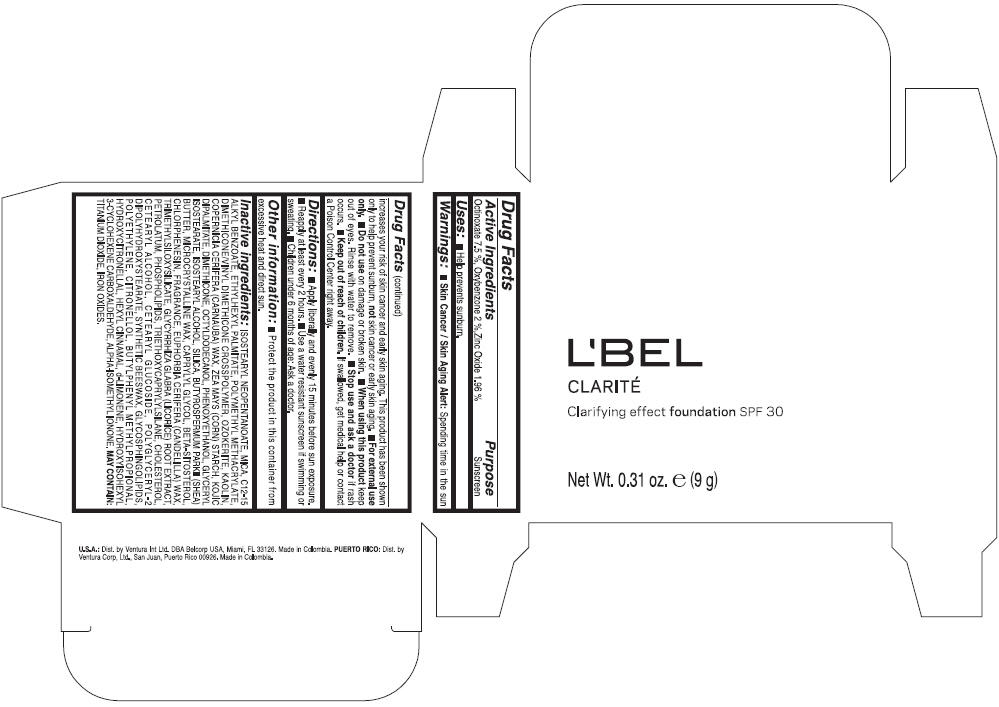
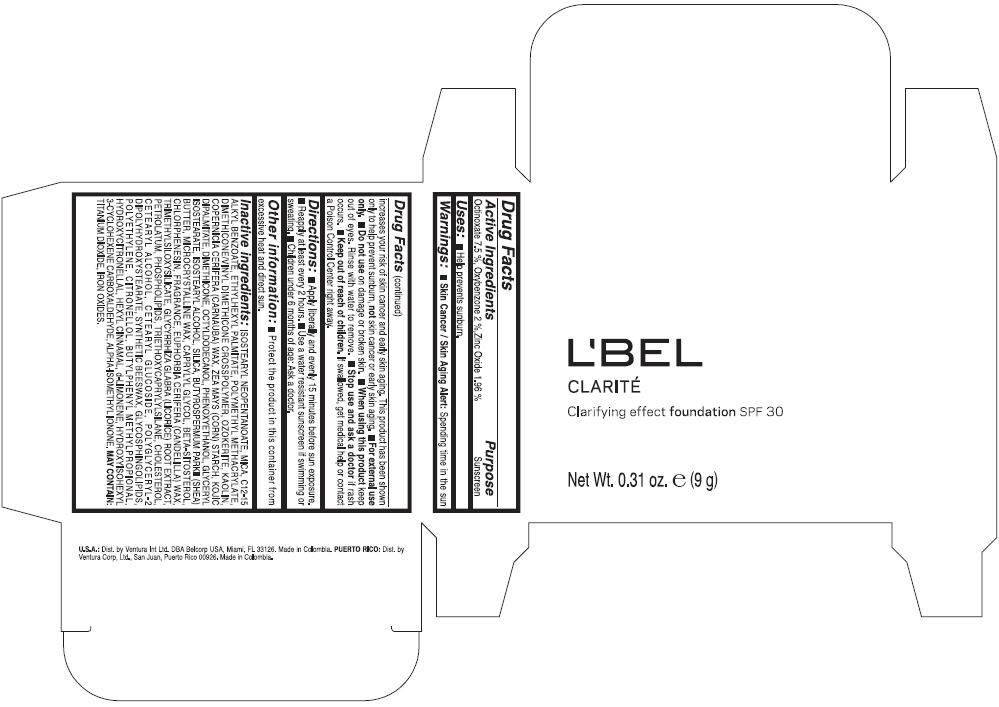
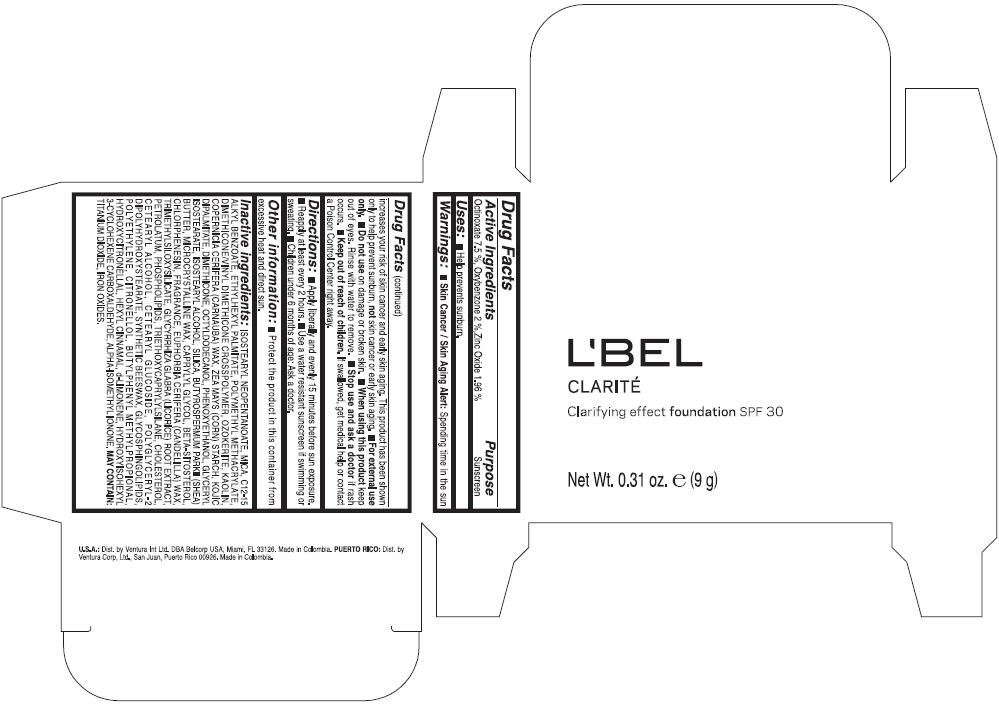
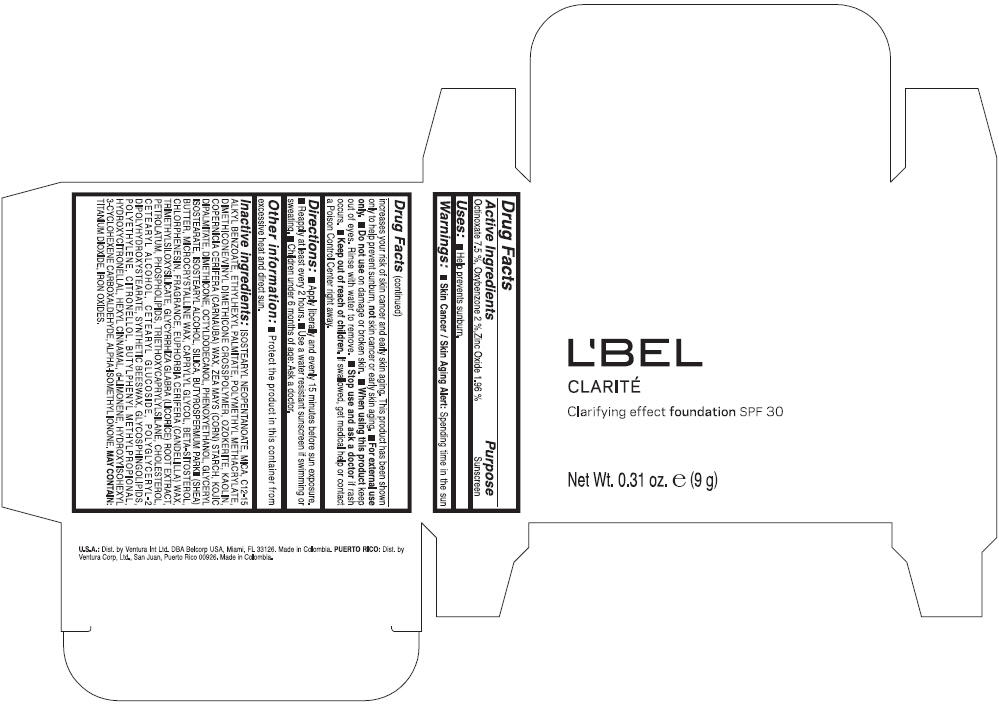
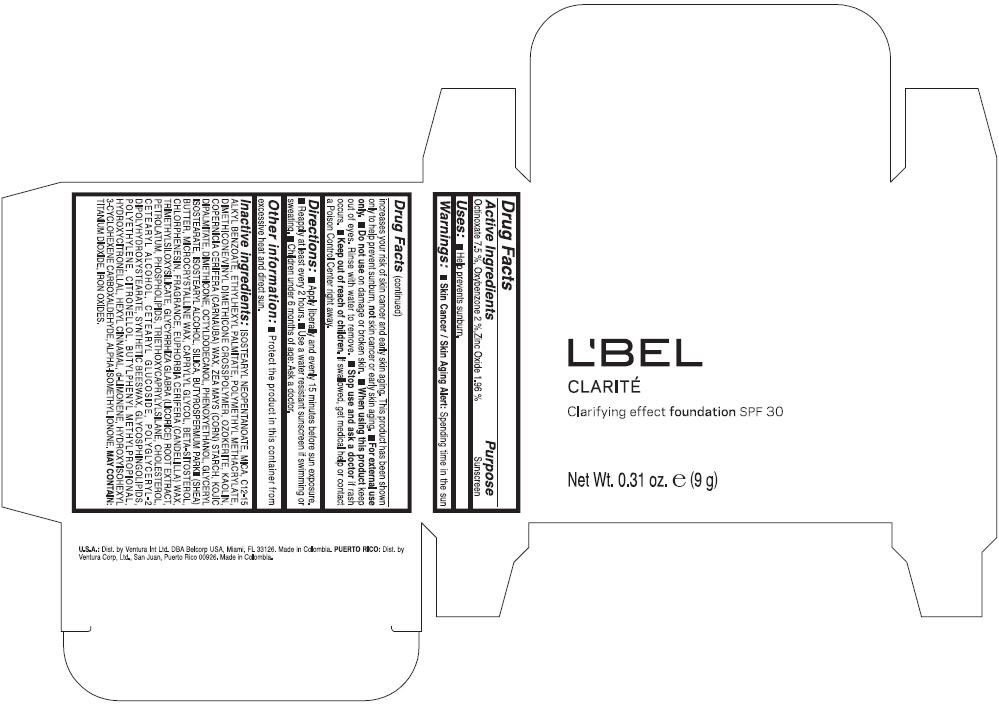
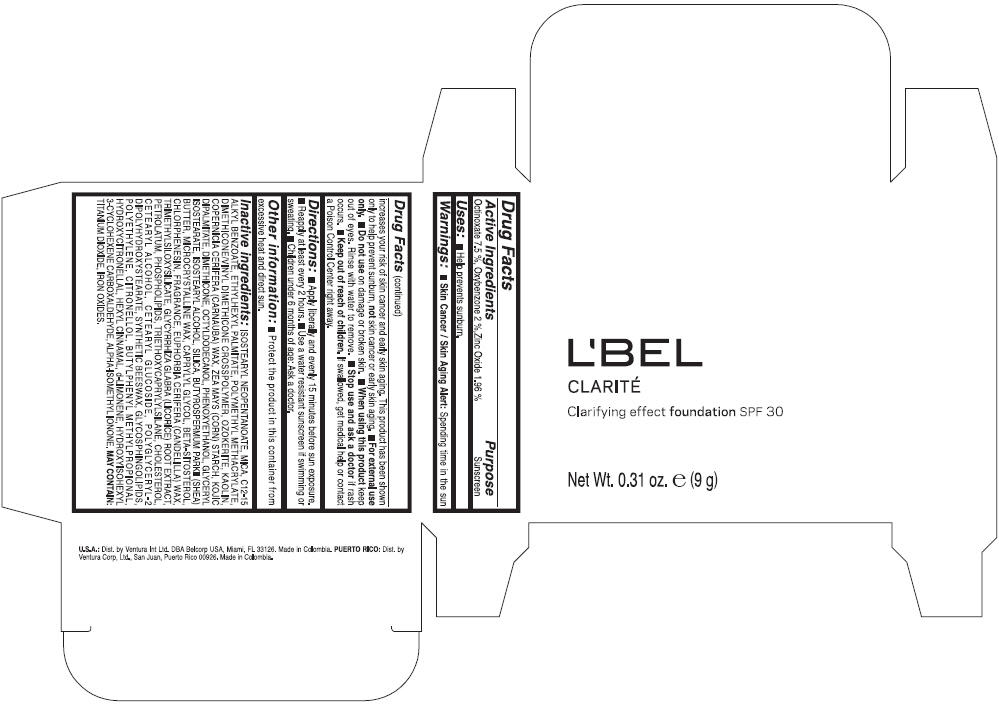
Label: LBEL CLARITE CLARIFYING EFFECT FOUNDATION SPF 30 CLAIRE 1- BEIGE- octinoxate, oxybenzone, and zinc oxide suspension
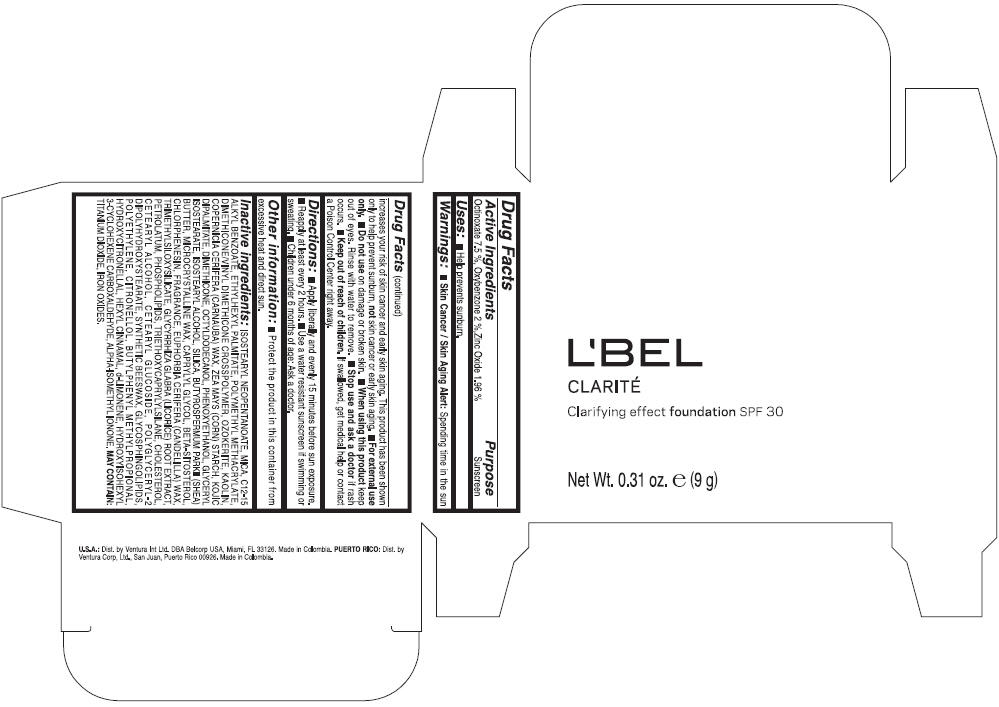
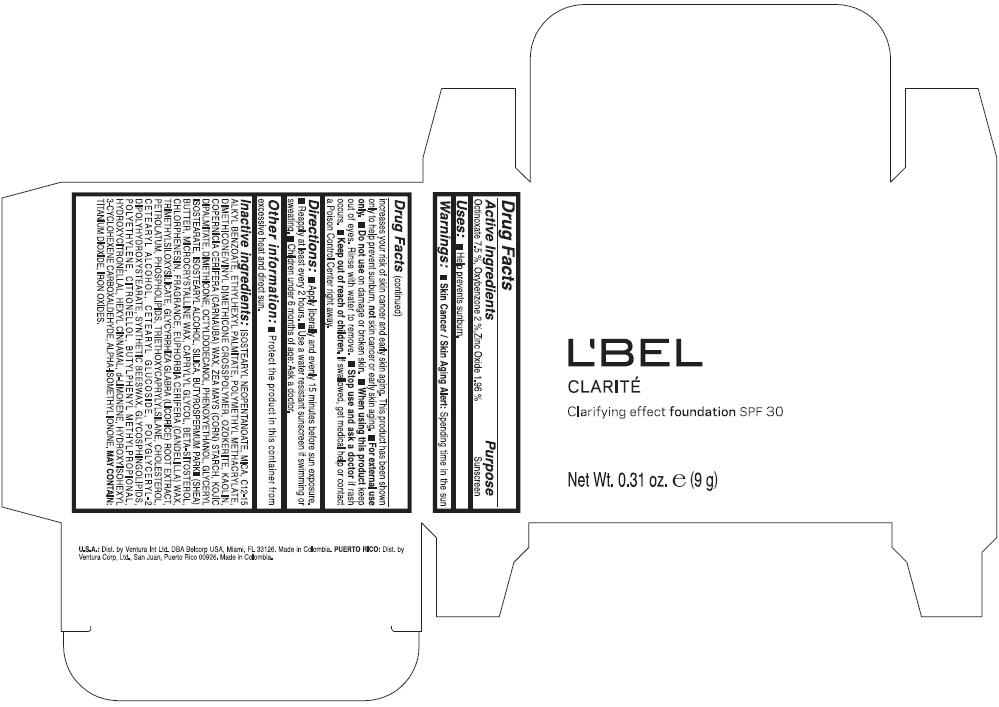
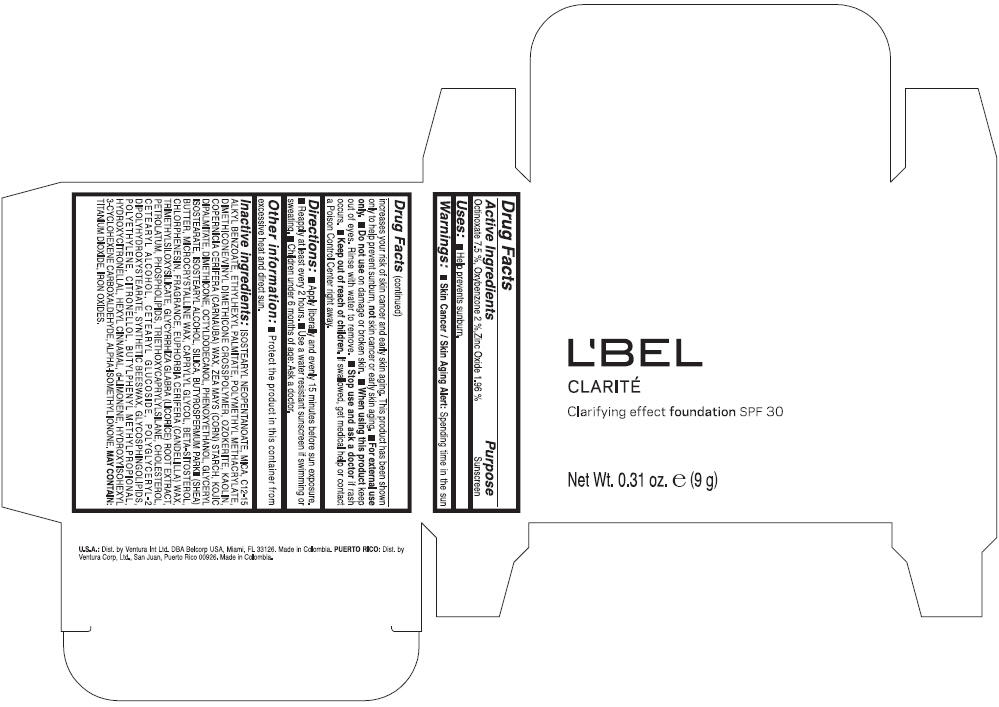
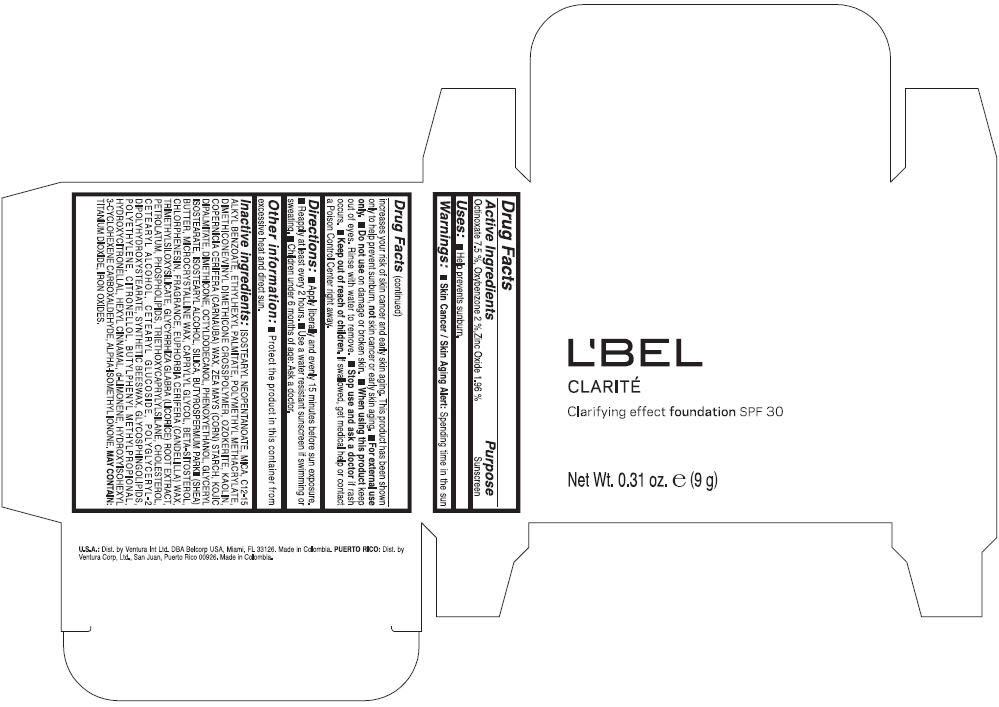
LBEL CLARITE CLARIFYING EFFECT FOUNDATION SPF 30 CLAIRE 2- BEIGE- octinoxate, oxybenzone, and zinc oxide suspension
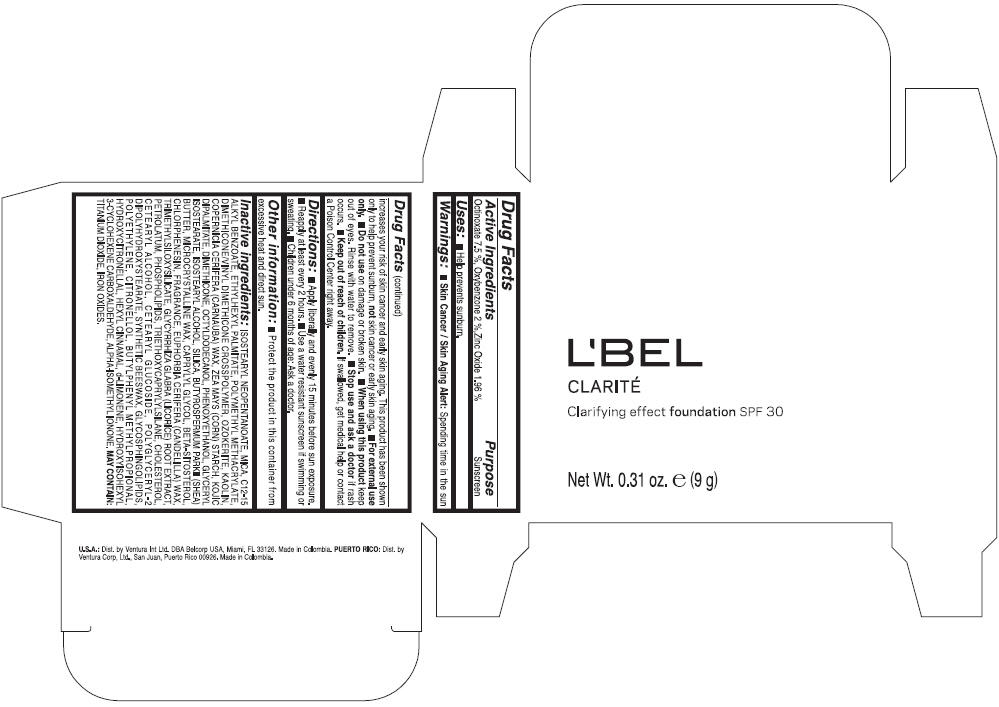
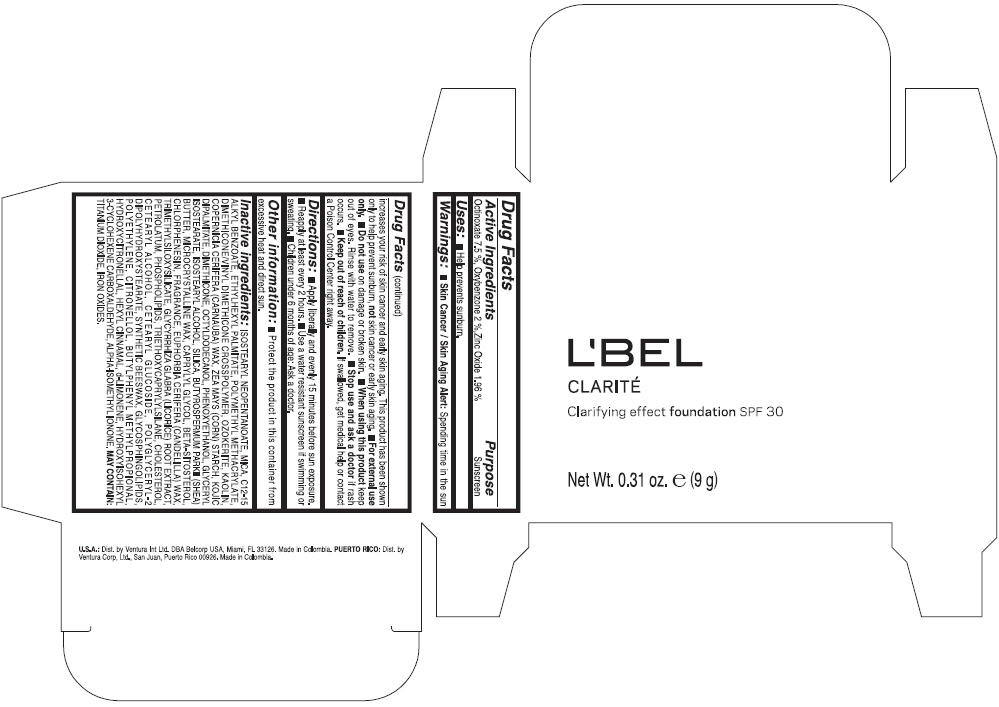
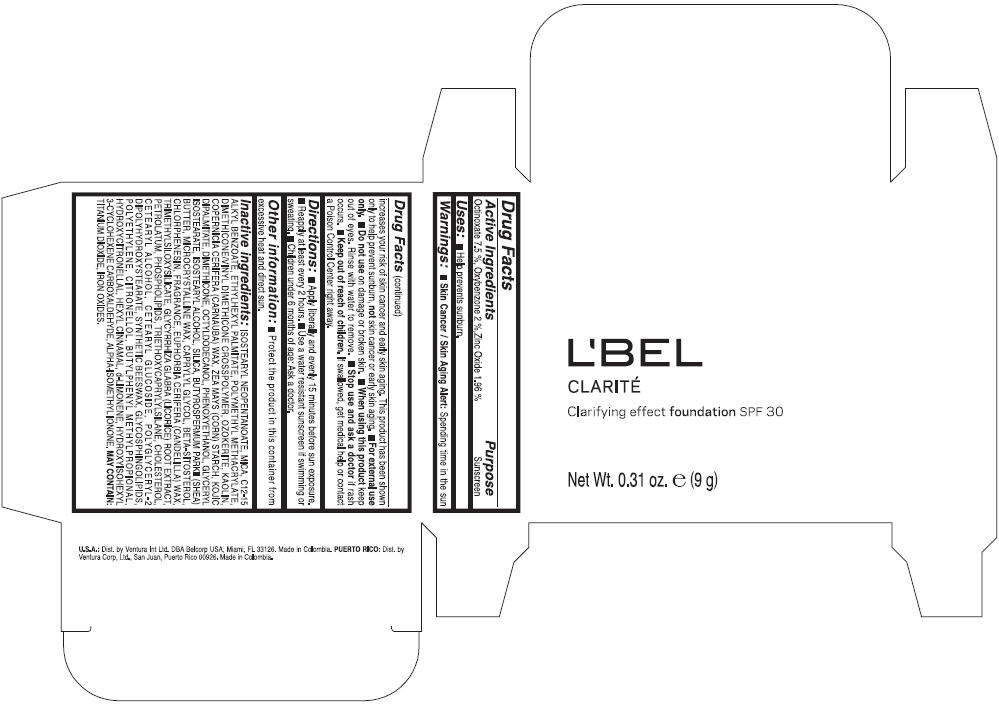
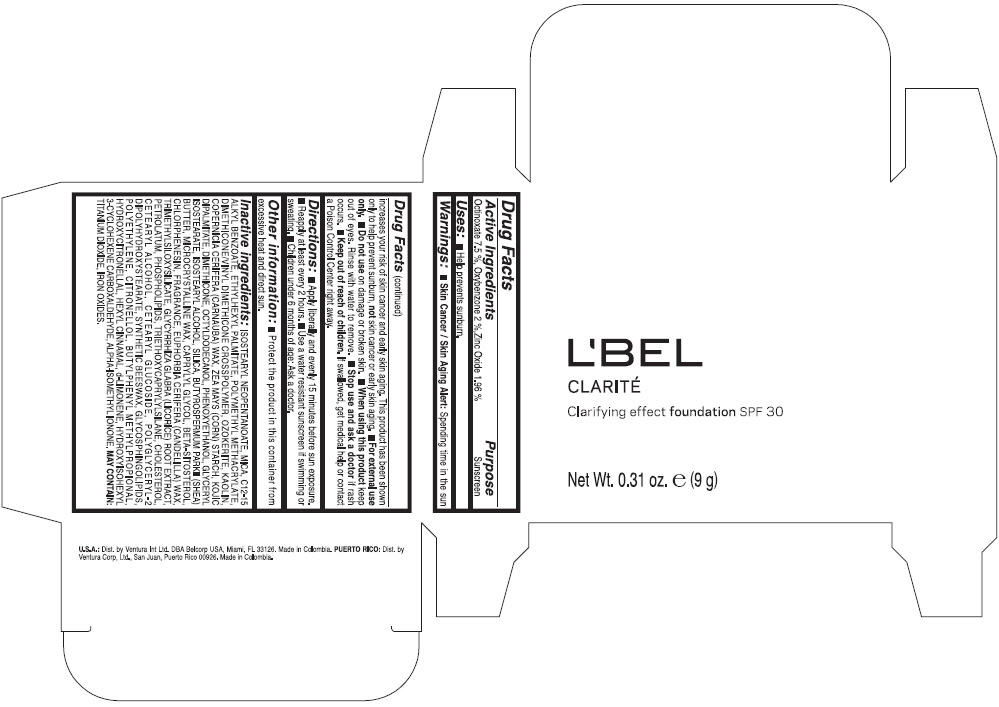
LBEL CLARITE CLARIFYING EFFECT FOUNDATION SPF 30 CLAIRE 3- BEIGE- octinoxate, oxybenzone, and zinc oxide suspension
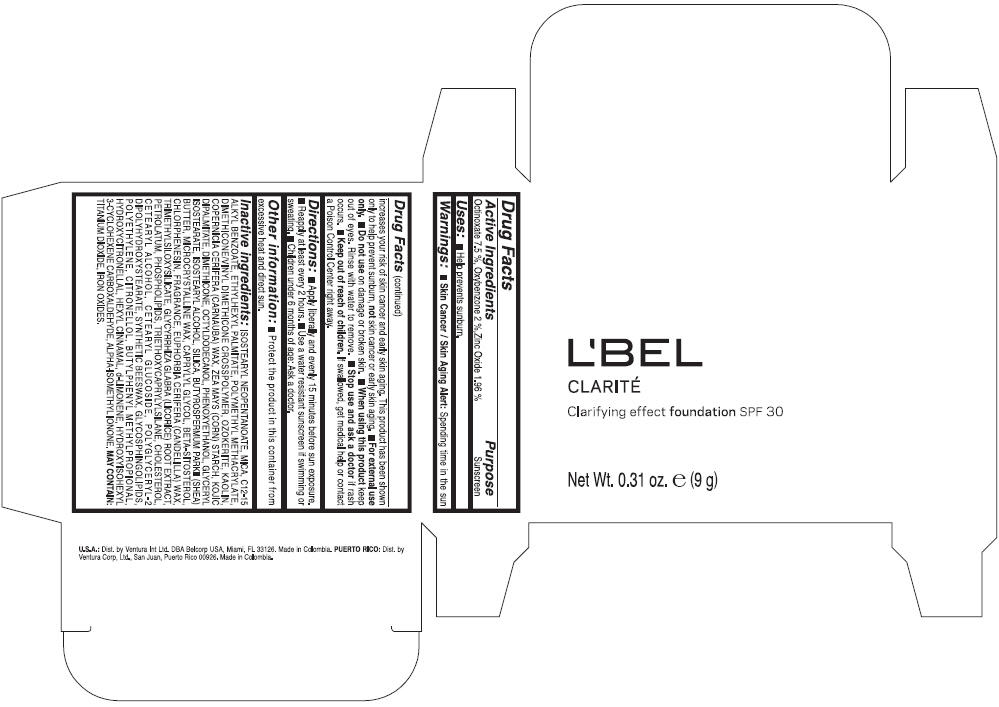
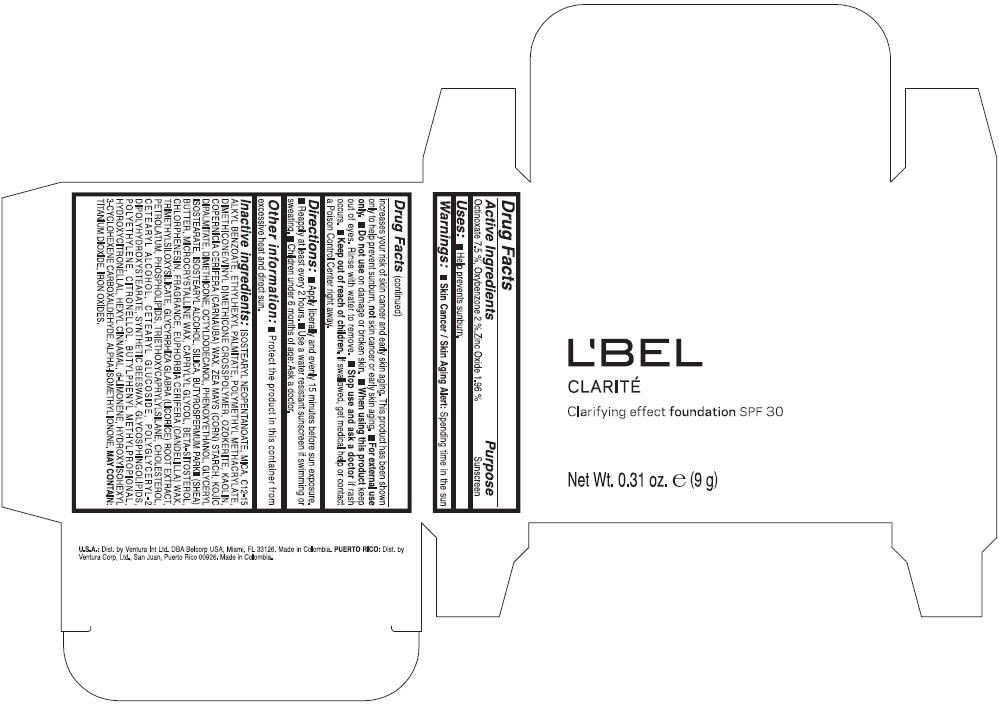
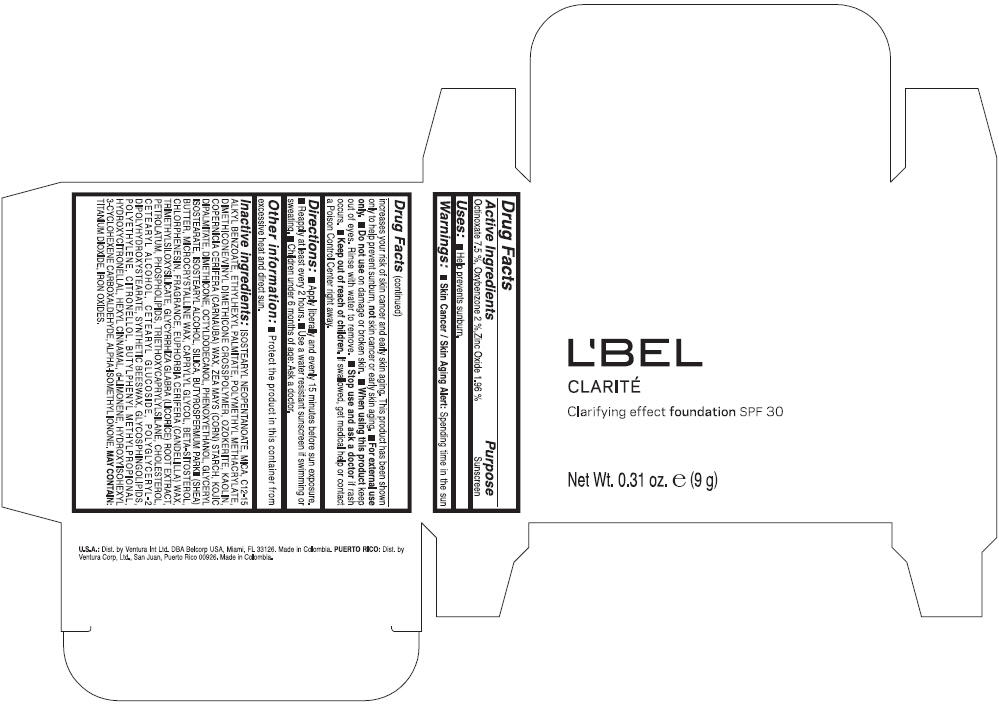
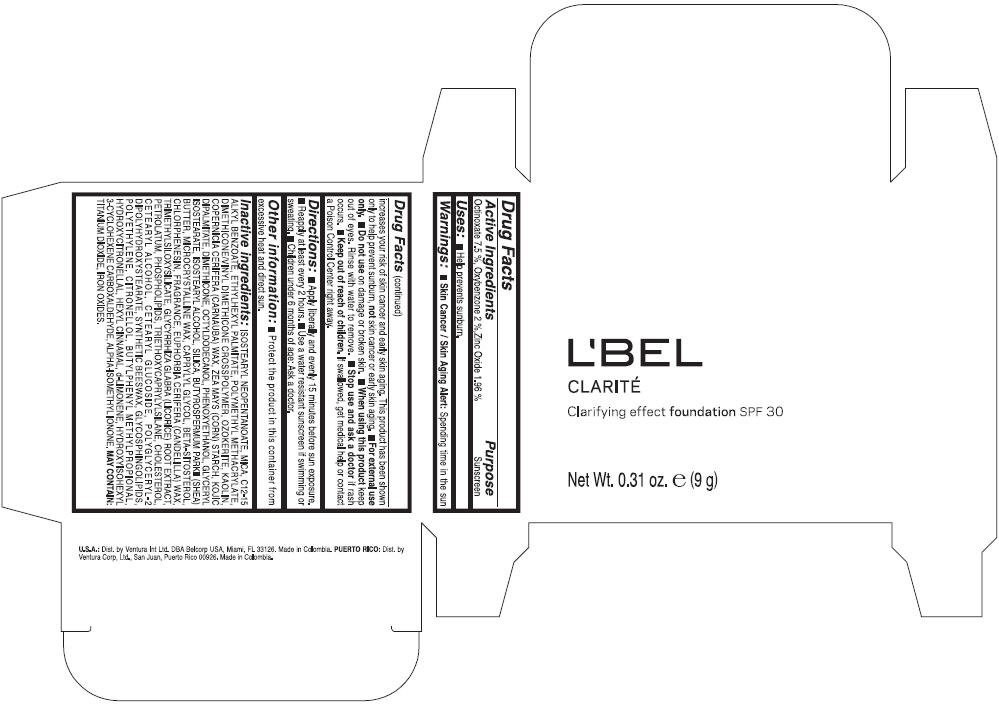
LBEL CLARITE CLARIFYING EFFECT FOUNDATION SPF 30 CLAIRE 4- BEIGE- octinoxate, oxybenzone, and zinc oxide suspension
LBEL CLARITE CLARIFYING EFFECT FOUNDATION SPF 30 MEDIUM 5- BEIGE- octinoxate, oxybenzone, and zinc oxide suspension
LBEL CLARITE CLARIFYING EFFECT FOUNDATION SPF 30 MEDIUM 6- BEIGE- octinoxate, oxybenzone, and zinc oxide suspension
LBEL CLARITE CLARIFYING EFFECT FOUNDATION SPF 30 MEDIUM 7- BEIGE- octinoxate, oxybenzone, and zinc oxide suspension
LBEL CLARITE CLARIFYING EFFECT FOUNDATION SPF 30 OBSCURE 8- BROWN- octinoxate, oxybenzone, and zinc oxide suspension
LBEL CLARITE CLARIFYING EFFECT FOUNDATION SPF 30 OBSCURE 9- BROWN- octinoxate, oxybenzone, and zinc oxide suspension
-
Contains inactivated NDC Code(s)
NDC Code(s): 13537-943-01, 13537-943-02, 13537-944-01, 13537-944-02, view more13537-945-01, 13537-945-02, 13537-946-01, 13537-946-02, 13537-947-01, 13537-947-02, 13537-948-01, 13537-948-02, 13537-949-01, 13537-949-02, 13537-950-01, 13537-950-02, 13537-951-01, 13537-951-02 - Packager: Ventura Corporation LTD
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated December 23, 2019
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- ACTIVE INGREDIENT
- Uses
- Warnings
- Directions
- Other information
-
Inactive ingredients
ISOSTEARYL NEOPENTANOATE, MICA, C12-15 ALKYL BENZOATE, ETHYLHEXYL PALMITATE, POLYMETHYL METHACRYLATE, DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER, OZOKERITE, KAOLIN, COPERNICIA CERIFERA CERA (COPERNICIA CERIFERA (CARNAUBA) WAX), ZEA MAYS STARCH (ZEA MAYS (CORN) STARCH), KOJIC DIPALMITATE, DIMETHICONE, OCTYLDODECANOL, PHENOXYETHANOL, GLYCERYL ISOSTEARATE, ISOSTEARYL ALCOHOL, SILICA, BUTYROSPERMUM PARKII BUTTER (BUTYROSPERMUM PARKII (SHEA) BUTTER), CERA MICROCRISTALLINA (MICROCRYSTALLINE WAX), CAPRYLYL GLYCOL, BETA-SITOSTEROL, CHLORPHENESIN, PARFUM (FRAGRANCE), CANDELILLA CERA (EUPHORBIA CERIFERA (CANDELILLA) WAX), TRIMETHYLSILOXYSILICATE, GLYCYRRHIZA GLABRA ROOT EXTRACT (GLYCYRRHIZA GLABRA (LICORICE) ROOT EXTRACT), PETROLATUM, PHOSPHOLIPIDS, TRIETHOXYCAPRYLYLSILANE, CHOLESTEROL, CETEARYL ALCOHOL, CETEARYL GLUCOSIDE, POLYGLYCERYL-2 DIPOLYHYDROXYSTEARATE, SYNTHETIC BEESWAX, GLYCOSPHINGOLIPIDS, POLYETHYLENE.
MAY CONTAIN : CI 77891 (TITANIUM DIOXIDE), CI 77492 (IRON OXIDES), CI 77491 (IRON OXIDES), CI 77499 (IRON OXIDES).
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 9 g Case Box - CLAIRE 1- BEIGE
- PRINCIPAL DISPLAY PANEL - 9 g Case Box - CLAIRE 2- BEIGE
- PRINCIPAL DISPLAY PANEL - 9 g Case Box - CLAIRE 3- BEIGE
- PRINCIPAL DISPLAY PANEL - 9 g Case Box - CLAIRE 4- BEIGE
- PRINCIPAL DISPLAY PANEL - 9 g Case Box - MEDIUM 5- BEIGE
- PRINCIPAL DISPLAY PANEL - 9 g Case Box - MEDIUM 6- BEIGE
- PRINCIPAL DISPLAY PANEL - 9 g Case Box - MEDIUM 7- BEIGE
- PRINCIPAL DISPLAY PANEL - 9 g Case Box - OBSCURE 8- BROWN
- PRINCIPAL DISPLAY PANEL - 9 g Case Box - OBSCURE 9- BROWN
- PRINCIPAL DISPLAY PANEL - Kit Label
-
INGREDIENTS AND APPEARANCE
LBEL CLARITE CLARIFYING EFFECT FOUNDATION SPF 30 CLAIRE 1- BEIGE
octinoxate, oxybenzone, and zinc oxide suspensionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:13537-943 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Octinoxate (UNII: 4Y5P7MUD51) (Octinoxate - UNII:4Y5P7MUD51) Octinoxate 0.075 g in 1 g Oxybenzone (UNII: 95OOS7VE0Y) (Oxybenzone - UNII:95OOS7VE0Y) Oxybenzone 0.02 g in 1 g Zinc Oxide (UNII: SOI2LOH54Z) (Zinc Oxide - UNII:SOI2LOH54Z) Zinc Oxide 0.0196 g in 1 g Inactive Ingredients Ingredient Name Strength ISOSTEARYL NEOPENTANOATE (UNII: 411THY156Q) MICA (UNII: V8A1AW0880) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) ETHYLHEXYL PALMITATE (UNII: 2865993309) POLY(METHYL METHACRYLATE; 450000 MW) (UNII: Z47NNT4J11) CERESIN (UNII: Q1LS2UJO3A) KAOLIN (UNII: 24H4NWX5CO) CARNAUBA WAX (UNII: R12CBM0EIZ) STARCH, CORN (UNII: O8232NY3SJ) KOJIC DIPALMITATE (UNII: 13N249RWTM) DIMETHICONE (UNII: 92RU3N3Y1O) OCTYLDODECANOL (UNII: 461N1O614Y) PHENOXYETHANOL (UNII: HIE492ZZ3T) GLYCERYL ISOSTEARATE (UNII: HYE7O27HAO) ISOSTEARYL ALCOHOL (UNII: Q613OCQ44Y) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SHEA BUTTER (UNII: K49155WL9Y) MICROCRYSTALLINE WAX (UNII: XOF597Q3KY) CAPRYLYL GLYCOL (UNII: 00YIU5438U) .BETA.-SITOSTEROL (UNII: S347WMO6M4) CHLORPHENESIN (UNII: I670DAL4SZ) CANDELILLA WAX (UNII: WL0328HX19) GLYCYRRHIZA GLABRA (UNII: 2788Z9758H) PETROLATUM (UNII: 4T6H12BN9U) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) CHOLESTEROL (UNII: 97C5T2UQ7J) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) CETEARYL GLUCOSIDE (UNII: 09FUA47KNA) POLYGLYCERYL-2 DIPOLYHYDROXYSTEARATE (UNII: 9229XJ4V12) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERRIC OXIDE RED (UNII: 1K09F3G675) FERROSOFERRIC OXIDE (UNII: XM0M87F357) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:13537-943-02 1 in 1 BOX 06/06/2016 1 NDC:13537-943-01 9 g in 1 CASE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 06/06/2016 LBEL CLARITE CLARIFYING EFFECT FOUNDATION SPF 30 CLAIRE 2- BEIGE
octinoxate, oxybenzone, and zinc oxide suspensionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:13537-944 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Octinoxate (UNII: 4Y5P7MUD51) (Octinoxate - UNII:4Y5P7MUD51) Octinoxate 0.075 g in 1 g Oxybenzone (UNII: 95OOS7VE0Y) (Oxybenzone - UNII:95OOS7VE0Y) Oxybenzone 0.02 g in 1 g Zinc Oxide (UNII: SOI2LOH54Z) (Zinc Oxide - UNII:SOI2LOH54Z) Zinc Oxide 0.0196 g in 1 g Inactive Ingredients Ingredient Name Strength ISOSTEARYL NEOPENTANOATE (UNII: 411THY156Q) MICA (UNII: V8A1AW0880) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) ETHYLHEXYL PALMITATE (UNII: 2865993309) POLY(METHYL METHACRYLATE; 450000 MW) (UNII: Z47NNT4J11) CERESIN (UNII: Q1LS2UJO3A) KAOLIN (UNII: 24H4NWX5CO) CARNAUBA WAX (UNII: R12CBM0EIZ) STARCH, CORN (UNII: O8232NY3SJ) KOJIC DIPALMITATE (UNII: 13N249RWTM) DIMETHICONE (UNII: 92RU3N3Y1O) OCTYLDODECANOL (UNII: 461N1O614Y) PHENOXYETHANOL (UNII: HIE492ZZ3T) GLYCERYL ISOSTEARATE (UNII: HYE7O27HAO) ISOSTEARYL ALCOHOL (UNII: Q613OCQ44Y) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SHEA BUTTER (UNII: K49155WL9Y) MICROCRYSTALLINE WAX (UNII: XOF597Q3KY) CAPRYLYL GLYCOL (UNII: 00YIU5438U) .BETA.-SITOSTEROL (UNII: S347WMO6M4) CHLORPHENESIN (UNII: I670DAL4SZ) CANDELILLA WAX (UNII: WL0328HX19) GLYCYRRHIZA GLABRA (UNII: 2788Z9758H) PETROLATUM (UNII: 4T6H12BN9U) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) CHOLESTEROL (UNII: 97C5T2UQ7J) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) CETEARYL GLUCOSIDE (UNII: 09FUA47KNA) POLYGLYCERYL-2 DIPOLYHYDROXYSTEARATE (UNII: 9229XJ4V12) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERRIC OXIDE RED (UNII: 1K09F3G675) FERROSOFERRIC OXIDE (UNII: XM0M87F357) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:13537-944-02 1 in 1 BOX 06/06/2016 1 NDC:13537-944-01 9 g in 1 CASE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 06/06/2016 LBEL CLARITE CLARIFYING EFFECT FOUNDATION SPF 30 CLAIRE 3- BEIGE
octinoxate, oxybenzone, and zinc oxide suspensionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:13537-945 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Octinoxate (UNII: 4Y5P7MUD51) (Octinoxate - UNII:4Y5P7MUD51) Octinoxate 0.075 g in 1 g Oxybenzone (UNII: 95OOS7VE0Y) (Oxybenzone - UNII:95OOS7VE0Y) Oxybenzone 0.02 g in 1 g Zinc Oxide (UNII: SOI2LOH54Z) (Zinc Oxide - UNII:SOI2LOH54Z) Zinc Oxide 0.0196 g in 1 g Inactive Ingredients Ingredient Name Strength ISOSTEARYL NEOPENTANOATE (UNII: 411THY156Q) MICA (UNII: V8A1AW0880) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) ETHYLHEXYL PALMITATE (UNII: 2865993309) POLY(METHYL METHACRYLATE; 450000 MW) (UNII: Z47NNT4J11) CERESIN (UNII: Q1LS2UJO3A) KAOLIN (UNII: 24H4NWX5CO) CARNAUBA WAX (UNII: R12CBM0EIZ) STARCH, CORN (UNII: O8232NY3SJ) KOJIC DIPALMITATE (UNII: 13N249RWTM) DIMETHICONE (UNII: 92RU3N3Y1O) OCTYLDODECANOL (UNII: 461N1O614Y) PHENOXYETHANOL (UNII: HIE492ZZ3T) GLYCERYL ISOSTEARATE (UNII: HYE7O27HAO) ISOSTEARYL ALCOHOL (UNII: Q613OCQ44Y) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SHEA BUTTER (UNII: K49155WL9Y) MICROCRYSTALLINE WAX (UNII: XOF597Q3KY) CAPRYLYL GLYCOL (UNII: 00YIU5438U) .BETA.-SITOSTEROL (UNII: S347WMO6M4) CHLORPHENESIN (UNII: I670DAL4SZ) CANDELILLA WAX (UNII: WL0328HX19) GLYCYRRHIZA GLABRA (UNII: 2788Z9758H) PETROLATUM (UNII: 4T6H12BN9U) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) CHOLESTEROL (UNII: 97C5T2UQ7J) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) CETEARYL GLUCOSIDE (UNII: 09FUA47KNA) POLYGLYCERYL-2 DIPOLYHYDROXYSTEARATE (UNII: 9229XJ4V12) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERRIC OXIDE RED (UNII: 1K09F3G675) FERROSOFERRIC OXIDE (UNII: XM0M87F357) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:13537-945-02 1 in 1 BOX 06/06/2016 1 NDC:13537-945-01 9 g in 1 CASE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 06/06/2016 LBEL CLARITE CLARIFYING EFFECT FOUNDATION SPF 30 CLAIRE 4- BEIGE
octinoxate, oxybenzone, and zinc oxide suspensionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:13537-946 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Octinoxate (UNII: 4Y5P7MUD51) (Octinoxate - UNII:4Y5P7MUD51) Octinoxate 0.075 g in 1 g Oxybenzone (UNII: 95OOS7VE0Y) (Oxybenzone - UNII:95OOS7VE0Y) Oxybenzone 0.02 g in 1 g Zinc Oxide (UNII: SOI2LOH54Z) (Zinc Oxide - UNII:SOI2LOH54Z) Zinc Oxide 0.0196 g in 1 g Inactive Ingredients Ingredient Name Strength ISOSTEARYL NEOPENTANOATE (UNII: 411THY156Q) MICA (UNII: V8A1AW0880) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) ETHYLHEXYL PALMITATE (UNII: 2865993309) POLY(METHYL METHACRYLATE; 450000 MW) (UNII: Z47NNT4J11) CERESIN (UNII: Q1LS2UJO3A) KAOLIN (UNII: 24H4NWX5CO) CARNAUBA WAX (UNII: R12CBM0EIZ) STARCH, CORN (UNII: O8232NY3SJ) KOJIC DIPALMITATE (UNII: 13N249RWTM) DIMETHICONE (UNII: 92RU3N3Y1O) OCTYLDODECANOL (UNII: 461N1O614Y) PHENOXYETHANOL (UNII: HIE492ZZ3T) GLYCERYL ISOSTEARATE (UNII: HYE7O27HAO) ISOSTEARYL ALCOHOL (UNII: Q613OCQ44Y) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SHEA BUTTER (UNII: K49155WL9Y) MICROCRYSTALLINE WAX (UNII: XOF597Q3KY) CAPRYLYL GLYCOL (UNII: 00YIU5438U) .BETA.-SITOSTEROL (UNII: S347WMO6M4) CHLORPHENESIN (UNII: I670DAL4SZ) CANDELILLA WAX (UNII: WL0328HX19) GLYCYRRHIZA GLABRA (UNII: 2788Z9758H) PETROLATUM (UNII: 4T6H12BN9U) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) CHOLESTEROL (UNII: 97C5T2UQ7J) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) CETEARYL GLUCOSIDE (UNII: 09FUA47KNA) POLYGLYCERYL-2 DIPOLYHYDROXYSTEARATE (UNII: 9229XJ4V12) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERRIC OXIDE RED (UNII: 1K09F3G675) FERROSOFERRIC OXIDE (UNII: XM0M87F357) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:13537-946-02 1 in 1 BOX 06/06/2016 1 NDC:13537-946-01 9 g in 1 CASE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 06/06/2016 LBEL CLARITE CLARIFYING EFFECT FOUNDATION SPF 30 MEDIUM 5- BEIGE
octinoxate, oxybenzone, and zinc oxide suspensionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:13537-947 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Octinoxate (UNII: 4Y5P7MUD51) (Octinoxate - UNII:4Y5P7MUD51) Octinoxate 0.075 g in 1 g Oxybenzone (UNII: 95OOS7VE0Y) (Oxybenzone - UNII:95OOS7VE0Y) Oxybenzone 0.02 g in 1 g Zinc Oxide (UNII: SOI2LOH54Z) (Zinc Oxide - UNII:SOI2LOH54Z) Zinc Oxide 0.0196 g in 1 g Inactive Ingredients Ingredient Name Strength ISOSTEARYL NEOPENTANOATE (UNII: 411THY156Q) MICA (UNII: V8A1AW0880) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) ETHYLHEXYL PALMITATE (UNII: 2865993309) POLY(METHYL METHACRYLATE; 450000 MW) (UNII: Z47NNT4J11) CERESIN (UNII: Q1LS2UJO3A) KAOLIN (UNII: 24H4NWX5CO) CARNAUBA WAX (UNII: R12CBM0EIZ) STARCH, CORN (UNII: O8232NY3SJ) KOJIC DIPALMITATE (UNII: 13N249RWTM) DIMETHICONE (UNII: 92RU3N3Y1O) OCTYLDODECANOL (UNII: 461N1O614Y) PHENOXYETHANOL (UNII: HIE492ZZ3T) GLYCERYL ISOSTEARATE (UNII: HYE7O27HAO) ISOSTEARYL ALCOHOL (UNII: Q613OCQ44Y) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SHEA BUTTER (UNII: K49155WL9Y) MICROCRYSTALLINE WAX (UNII: XOF597Q3KY) CAPRYLYL GLYCOL (UNII: 00YIU5438U) .BETA.-SITOSTEROL (UNII: S347WMO6M4) CHLORPHENESIN (UNII: I670DAL4SZ) CANDELILLA WAX (UNII: WL0328HX19) GLYCYRRHIZA GLABRA (UNII: 2788Z9758H) PETROLATUM (UNII: 4T6H12BN9U) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) CHOLESTEROL (UNII: 97C5T2UQ7J) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) CETEARYL GLUCOSIDE (UNII: 09FUA47KNA) POLYGLYCERYL-2 DIPOLYHYDROXYSTEARATE (UNII: 9229XJ4V12) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERRIC OXIDE RED (UNII: 1K09F3G675) FERROSOFERRIC OXIDE (UNII: XM0M87F357) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:13537-947-02 1 in 1 BOX 06/06/2016 1 NDC:13537-947-01 9 g in 1 CASE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 06/06/2016 LBEL CLARITE CLARIFYING EFFECT FOUNDATION SPF 30 MEDIUM 6- BEIGE
octinoxate, oxybenzone, and zinc oxide suspensionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:13537-948 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Octinoxate (UNII: 4Y5P7MUD51) (Octinoxate - UNII:4Y5P7MUD51) Octinoxate 0.075 g in 1 g Oxybenzone (UNII: 95OOS7VE0Y) (Oxybenzone - UNII:95OOS7VE0Y) Oxybenzone 0.02 g in 1 g Zinc Oxide (UNII: SOI2LOH54Z) (Zinc Oxide - UNII:SOI2LOH54Z) Zinc Oxide 0.0196 g in 1 g Inactive Ingredients Ingredient Name Strength ISOSTEARYL NEOPENTANOATE (UNII: 411THY156Q) MICA (UNII: V8A1AW0880) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) ETHYLHEXYL PALMITATE (UNII: 2865993309) POLY(METHYL METHACRYLATE; 450000 MW) (UNII: Z47NNT4J11) CERESIN (UNII: Q1LS2UJO3A) KAOLIN (UNII: 24H4NWX5CO) CARNAUBA WAX (UNII: R12CBM0EIZ) STARCH, CORN (UNII: O8232NY3SJ) KOJIC DIPALMITATE (UNII: 13N249RWTM) DIMETHICONE (UNII: 92RU3N3Y1O) OCTYLDODECANOL (UNII: 461N1O614Y) PHENOXYETHANOL (UNII: HIE492ZZ3T) GLYCERYL ISOSTEARATE (UNII: HYE7O27HAO) ISOSTEARYL ALCOHOL (UNII: Q613OCQ44Y) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SHEA BUTTER (UNII: K49155WL9Y) MICROCRYSTALLINE WAX (UNII: XOF597Q3KY) CAPRYLYL GLYCOL (UNII: 00YIU5438U) .BETA.-SITOSTEROL (UNII: S347WMO6M4) CHLORPHENESIN (UNII: I670DAL4SZ) CANDELILLA WAX (UNII: WL0328HX19) GLYCYRRHIZA GLABRA (UNII: 2788Z9758H) PETROLATUM (UNII: 4T6H12BN9U) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) CHOLESTEROL (UNII: 97C5T2UQ7J) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) CETEARYL GLUCOSIDE (UNII: 09FUA47KNA) POLYGLYCERYL-2 DIPOLYHYDROXYSTEARATE (UNII: 9229XJ4V12) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERRIC OXIDE RED (UNII: 1K09F3G675) FERROSOFERRIC OXIDE (UNII: XM0M87F357) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:13537-948-02 1 in 1 BOX 06/06/2016 1 NDC:13537-948-01 9 g in 1 CASE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 06/06/2016 LBEL CLARITE CLARIFYING EFFECT FOUNDATION SPF 30 MEDIUM 7- BEIGE
octinoxate, oxybenzone, and zinc oxide suspensionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:13537-949 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Octinoxate (UNII: 4Y5P7MUD51) (Octinoxate - UNII:4Y5P7MUD51) Octinoxate 0.075 g in 1 g Oxybenzone (UNII: 95OOS7VE0Y) (Oxybenzone - UNII:95OOS7VE0Y) Oxybenzone 0.02 g in 1 g Zinc Oxide (UNII: SOI2LOH54Z) (Zinc Oxide - UNII:SOI2LOH54Z) Zinc Oxide 0.0196 g in 1 g Inactive Ingredients Ingredient Name Strength ISOSTEARYL NEOPENTANOATE (UNII: 411THY156Q) MICA (UNII: V8A1AW0880) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) ETHYLHEXYL PALMITATE (UNII: 2865993309) POLY(METHYL METHACRYLATE; 450000 MW) (UNII: Z47NNT4J11) CERESIN (UNII: Q1LS2UJO3A) KAOLIN (UNII: 24H4NWX5CO) CARNAUBA WAX (UNII: R12CBM0EIZ) STARCH, CORN (UNII: O8232NY3SJ) KOJIC DIPALMITATE (UNII: 13N249RWTM) DIMETHICONE (UNII: 92RU3N3Y1O) OCTYLDODECANOL (UNII: 461N1O614Y) PHENOXYETHANOL (UNII: HIE492ZZ3T) GLYCERYL ISOSTEARATE (UNII: HYE7O27HAO) ISOSTEARYL ALCOHOL (UNII: Q613OCQ44Y) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SHEA BUTTER (UNII: K49155WL9Y) MICROCRYSTALLINE WAX (UNII: XOF597Q3KY) CAPRYLYL GLYCOL (UNII: 00YIU5438U) .BETA.-SITOSTEROL (UNII: S347WMO6M4) CHLORPHENESIN (UNII: I670DAL4SZ) CANDELILLA WAX (UNII: WL0328HX19) GLYCYRRHIZA GLABRA (UNII: 2788Z9758H) PETROLATUM (UNII: 4T6H12BN9U) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) CHOLESTEROL (UNII: 97C5T2UQ7J) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) CETEARYL GLUCOSIDE (UNII: 09FUA47KNA) POLYGLYCERYL-2 DIPOLYHYDROXYSTEARATE (UNII: 9229XJ4V12) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERRIC OXIDE RED (UNII: 1K09F3G675) FERROSOFERRIC OXIDE (UNII: XM0M87F357) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:13537-949-02 1 in 1 BOX 06/06/2016 1 NDC:13537-949-01 9 g in 1 CASE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 06/06/2016 LBEL CLARITE CLARIFYING EFFECT FOUNDATION SPF 30 OBSCURE 8- BROWN
octinoxate, oxybenzone, and zinc oxide suspensionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:13537-950 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Octinoxate (UNII: 4Y5P7MUD51) (Octinoxate - UNII:4Y5P7MUD51) Octinoxate 0.075 g in 1 g Oxybenzone (UNII: 95OOS7VE0Y) (Oxybenzone - UNII:95OOS7VE0Y) Oxybenzone 0.02 g in 1 g Zinc Oxide (UNII: SOI2LOH54Z) (Zinc Oxide - UNII:SOI2LOH54Z) Zinc Oxide 0.0196 g in 1 g Inactive Ingredients Ingredient Name Strength ISOSTEARYL NEOPENTANOATE (UNII: 411THY156Q) MICA (UNII: V8A1AW0880) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) ETHYLHEXYL PALMITATE (UNII: 2865993309) POLY(METHYL METHACRYLATE; 450000 MW) (UNII: Z47NNT4J11) CERESIN (UNII: Q1LS2UJO3A) KAOLIN (UNII: 24H4NWX5CO) CARNAUBA WAX (UNII: R12CBM0EIZ) STARCH, CORN (UNII: O8232NY3SJ) KOJIC DIPALMITATE (UNII: 13N249RWTM) DIMETHICONE (UNII: 92RU3N3Y1O) OCTYLDODECANOL (UNII: 461N1O614Y) PHENOXYETHANOL (UNII: HIE492ZZ3T) GLYCERYL ISOSTEARATE (UNII: HYE7O27HAO) ISOSTEARYL ALCOHOL (UNII: Q613OCQ44Y) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SHEA BUTTER (UNII: K49155WL9Y) MICROCRYSTALLINE WAX (UNII: XOF597Q3KY) CAPRYLYL GLYCOL (UNII: 00YIU5438U) .BETA.-SITOSTEROL (UNII: S347WMO6M4) CHLORPHENESIN (UNII: I670DAL4SZ) CANDELILLA WAX (UNII: WL0328HX19) GLYCYRRHIZA GLABRA (UNII: 2788Z9758H) PETROLATUM (UNII: 4T6H12BN9U) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) CHOLESTEROL (UNII: 97C5T2UQ7J) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) CETEARYL GLUCOSIDE (UNII: 09FUA47KNA) POLYGLYCERYL-2 DIPOLYHYDROXYSTEARATE (UNII: 9229XJ4V12) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERRIC OXIDE RED (UNII: 1K09F3G675) FERROSOFERRIC OXIDE (UNII: XM0M87F357) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:13537-950-02 1 in 1 BOX 06/06/2016 1 NDC:13537-950-01 9 g in 1 CASE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 06/06/2016 LBEL CLARITE CLARIFYING EFFECT FOUNDATION SPF 30 OBSCURE 9- BROWN
octinoxate, oxybenzone, and zinc oxide suspensionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:13537-951 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Octinoxate (UNII: 4Y5P7MUD51) (Octinoxate - UNII:4Y5P7MUD51) Octinoxate 0.075 g in 1 g Oxybenzone (UNII: 95OOS7VE0Y) (Oxybenzone - UNII:95OOS7VE0Y) Oxybenzone 0.02 g in 1 g Zinc Oxide (UNII: SOI2LOH54Z) (Zinc Oxide - UNII:SOI2LOH54Z) Zinc Oxide 0.0196 g in 1 g Inactive Ingredients Ingredient Name Strength ISOSTEARYL NEOPENTANOATE (UNII: 411THY156Q) MICA (UNII: V8A1AW0880) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) ETHYLHEXYL PALMITATE (UNII: 2865993309) POLY(METHYL METHACRYLATE; 450000 MW) (UNII: Z47NNT4J11) CERESIN (UNII: Q1LS2UJO3A) KAOLIN (UNII: 24H4NWX5CO) CARNAUBA WAX (UNII: R12CBM0EIZ) STARCH, CORN (UNII: O8232NY3SJ) KOJIC DIPALMITATE (UNII: 13N249RWTM) DIMETHICONE (UNII: 92RU3N3Y1O) OCTYLDODECANOL (UNII: 461N1O614Y) PHENOXYETHANOL (UNII: HIE492ZZ3T) GLYCERYL ISOSTEARATE (UNII: HYE7O27HAO) ISOSTEARYL ALCOHOL (UNII: Q613OCQ44Y) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SHEA BUTTER (UNII: K49155WL9Y) MICROCRYSTALLINE WAX (UNII: XOF597Q3KY) CAPRYLYL GLYCOL (UNII: 00YIU5438U) .BETA.-SITOSTEROL (UNII: S347WMO6M4) CHLORPHENESIN (UNII: I670DAL4SZ) CANDELILLA WAX (UNII: WL0328HX19) GLYCYRRHIZA GLABRA (UNII: 2788Z9758H) PETROLATUM (UNII: 4T6H12BN9U) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) CHOLESTEROL (UNII: 97C5T2UQ7J) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) CETEARYL GLUCOSIDE (UNII: 09FUA47KNA) POLYGLYCERYL-2 DIPOLYHYDROXYSTEARATE (UNII: 9229XJ4V12) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERRIC OXIDE RED (UNII: 1K09F3G675) FERROSOFERRIC OXIDE (UNII: XM0M87F357) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:13537-951-02 1 in 1 BOX 06/06/2016 1 NDC:13537-951-01 9 g in 1 CASE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 06/06/2016 Labeler - Ventura Corporation LTD (602751344) Establishment Name Address ID/FEI Business Operations Bel Star S.A. (Colombia) 880160197 MANUFACTURE(13537-943, 13537-944, 13537-945, 13537-946, 13537-947, 13537-948, 13537-949, 13537-950, 13537-951)