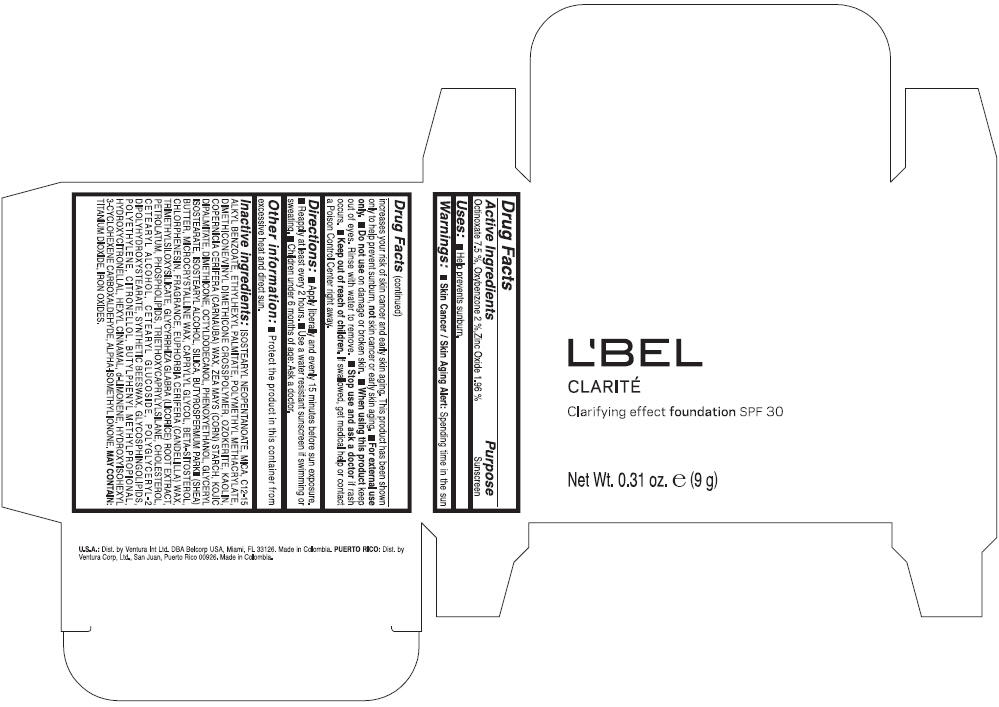
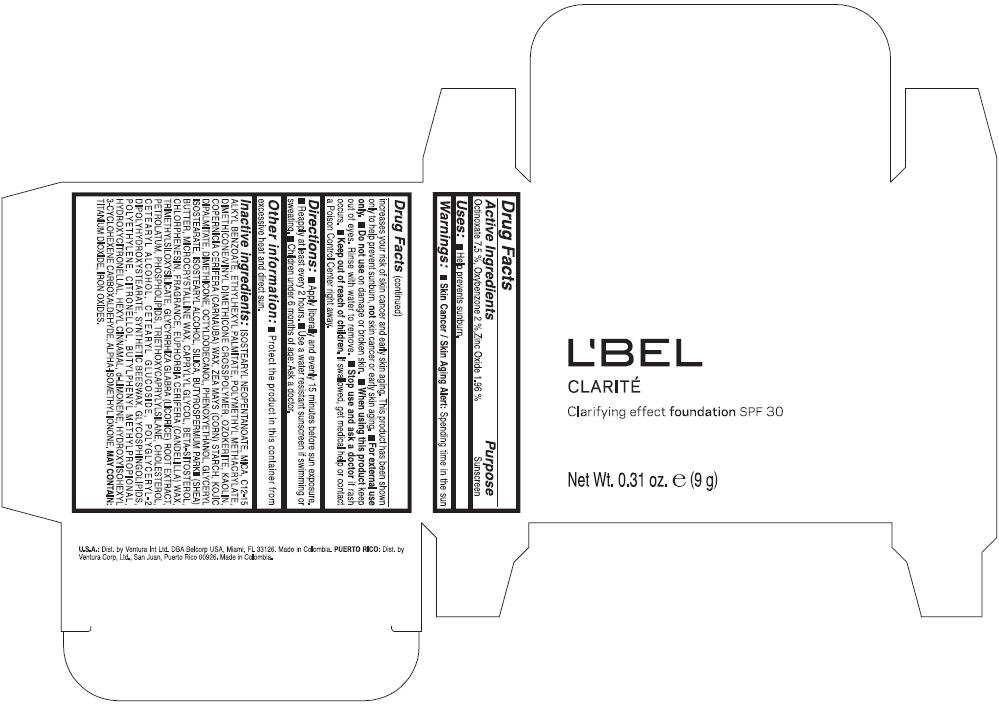
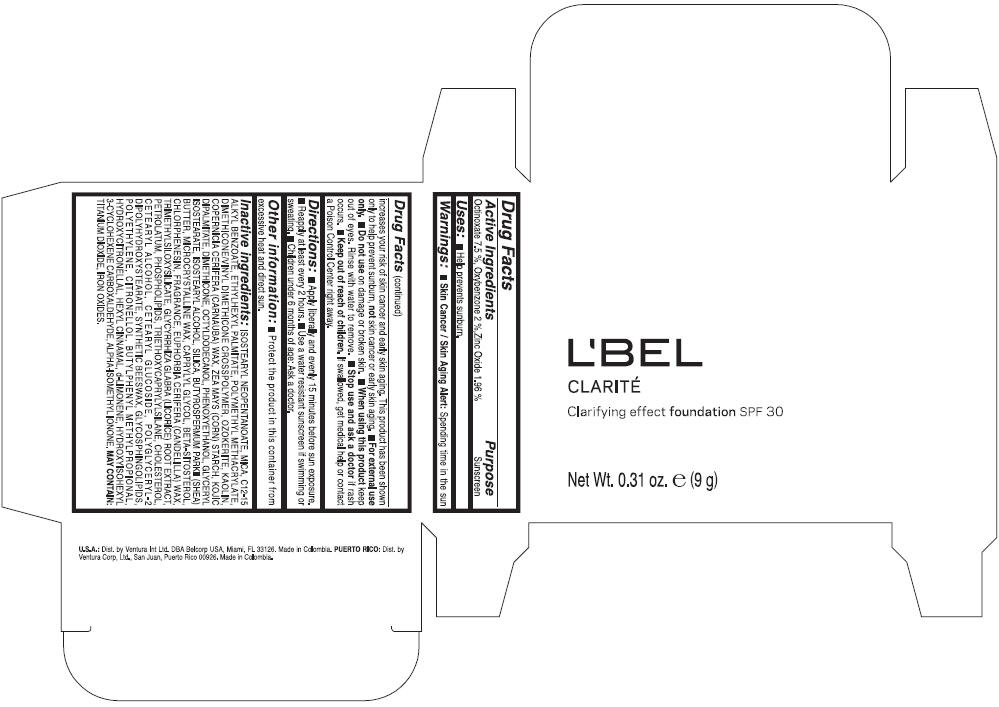
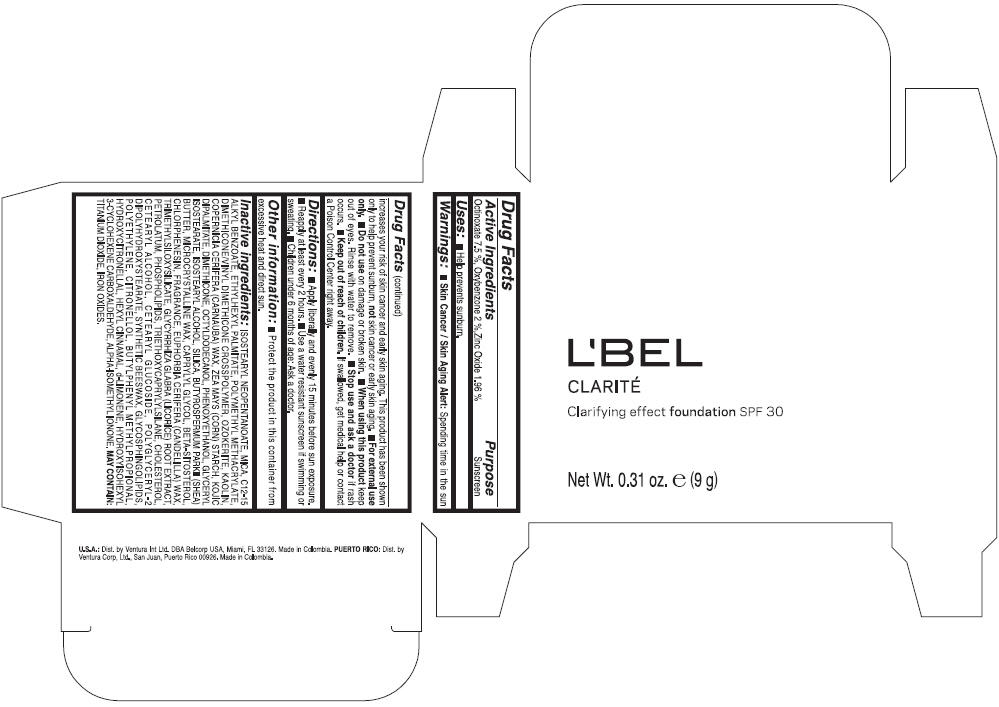
Warnings
Directions
- Apply liberally and evenly 15 minutes before sun exposure.
- Reapply at least every 2 hours
- Use a water resistant sunscreen if swimming or sweating.
- Children under 6 months of age: Ask a doctor
Inactive ingredients
ISOSTEARYL NEOPENTANOATE, MICA, C12-15 ALKYL BENZOATE, ETHYLHEXYL PALMITATE, POLYMETHYL METHACRYLATE, DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER, OZOKERITE, KAOLIN, COPERNICIA CERIFERA CERA (COPERNICIA CERIFERA (CARNAUBA) WAX), ZEA MAYS STARCH (ZEA MAYS (CORN) STARCH), KOJIC DIPALMITATE, DIMETHICONE, OCTYLDODECANOL, PHENOXYETHANOL, GLYCERYL ISOSTEARATE, ISOSTEARYL ALCOHOL, SILICA, BUTYROSPERMUM PARKII BUTTER (BUTYROSPERMUM PARKII (SHEA) BUTTER), CERA MICROCRISTALLINA (MICROCRYSTALLINE WAX), CAPRYLYL GLYCOL, BETA-SITOSTEROL, CHLORPHENESIN, PARFUM (FRAGRANCE), CANDELILLA CERA (EUPHORBIA CERIFERA (CANDELILLA) WAX), TRIMETHYLSILOXYSILICATE, GLYCYRRHIZA GLABRA ROOT EXTRACT (GLYCYRRHIZA GLABRA (LICORICE) ROOT EXTRACT), PETROLATUM, PHOSPHOLIPIDS, TRIETHOXYCAPRYLYLSILANE, CHOLESTEROL, CETEARYL ALCOHOL, CETEARYL GLUCOSIDE, POLYGLYCERYL-2 DIPOLYHYDROXYSTEARATE, SYNTHETIC BEESWAX, GLYCOSPHINGOLIPIDS, POLYETHYLENE.
MAY CONTAIN : CI 77891 (TITANIUM DIOXIDE), CI 77492 (IRON OXIDES), CI 77491 (IRON OXIDES), CI 77499 (IRON OXIDES).
PRINCIPAL DISPLAY PANEL - 9 g Case Box - CLAIRE 1- BEIGE
L'BEL
CLARITÉ
Clarifying effect foundation SPF 30
Net Wt. 0.31 oz. e (9 g)
PRINCIPAL DISPLAY PANEL - 9 g Case Box - CLAIRE 2- BEIGE
L'BEL
CLARITÉ
Clarifying effect foundation SPF 30
Net Wt. 0.31 oz. e (9 g)
PRINCIPAL DISPLAY PANEL - 9 g Case Box - CLAIRE 3- BEIGE
L'BEL
CLARITÉ
Clarifying effect foundation SPF 30
Net Wt. 0.31 oz. e (9 g)
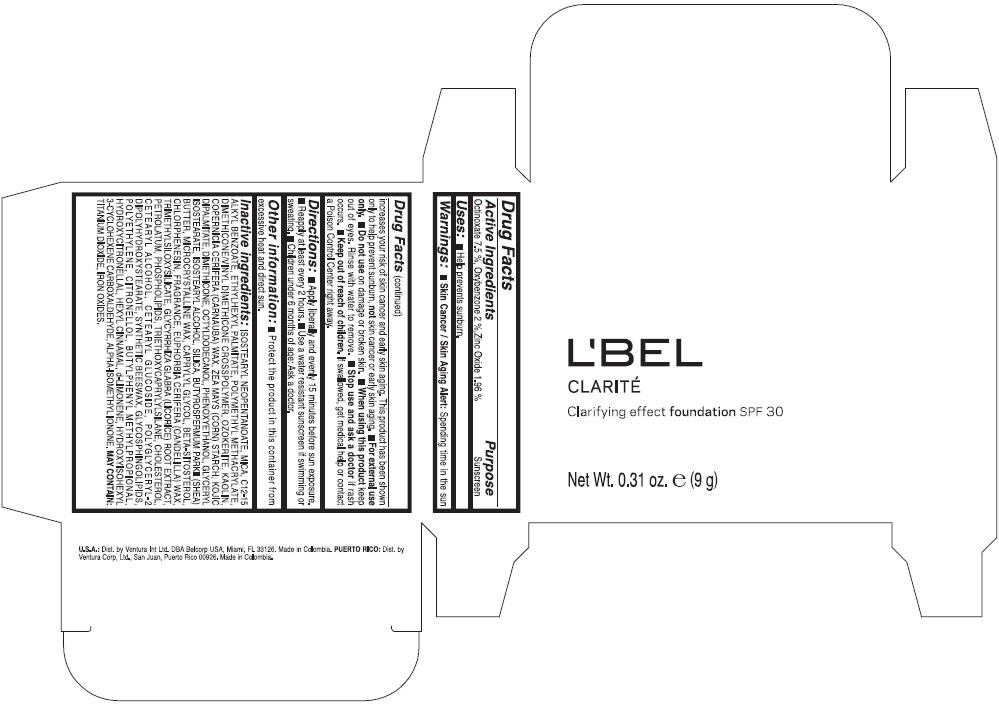
PRINCIPAL DISPLAY PANEL - 9 g Case Box - CLAIRE 4- BEIGE
L'BEL
CLARITÉ
Clarifying effect foundation SPF 30
Net Wt. 0.31 oz. e (9 g)
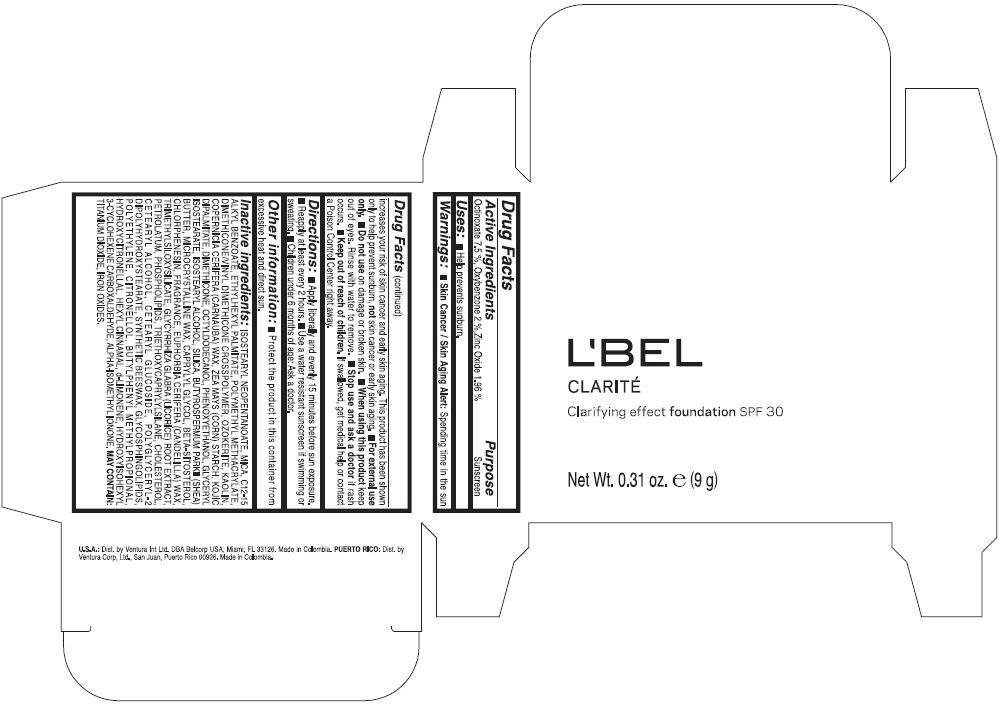
PRINCIPAL DISPLAY PANEL - 9 g Case Box - MEDIUM 5- BEIGE
L'BEL
CLARITÉ
Clarifying effect foundation SPF 30
Net Wt. 0.31 oz. e (9 g)
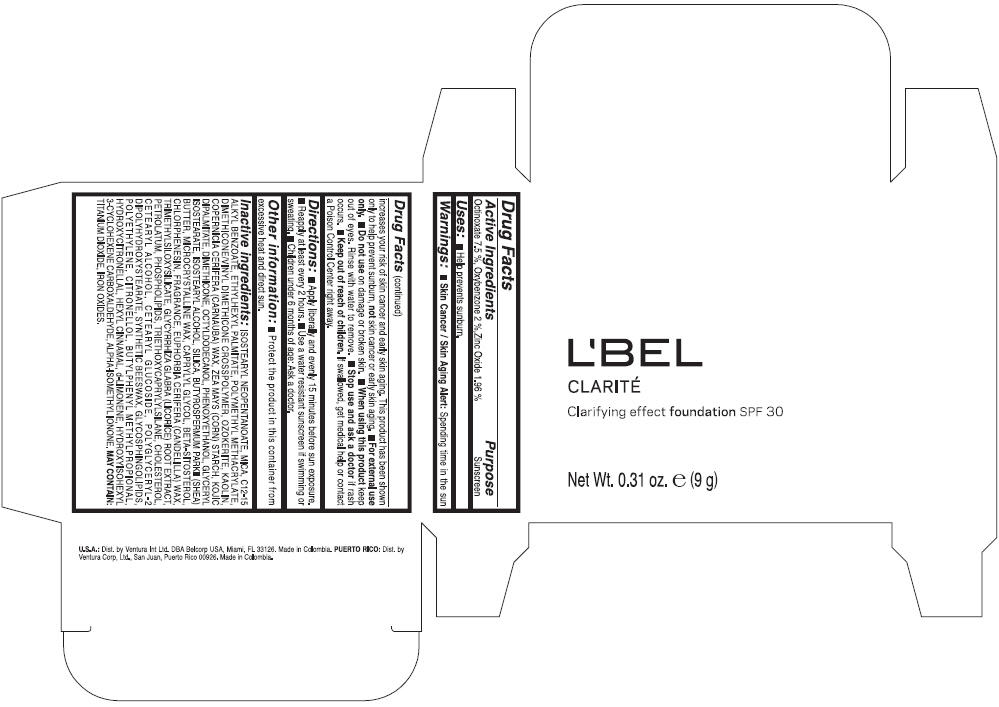
PRINCIPAL DISPLAY PANEL - 9 g Case Box - MEDIUM 6- BEIGE
L'BEL
CLARITÉ
Clarifying effect foundation SPF 30
Net Wt. 0.31 oz. e (9 g)
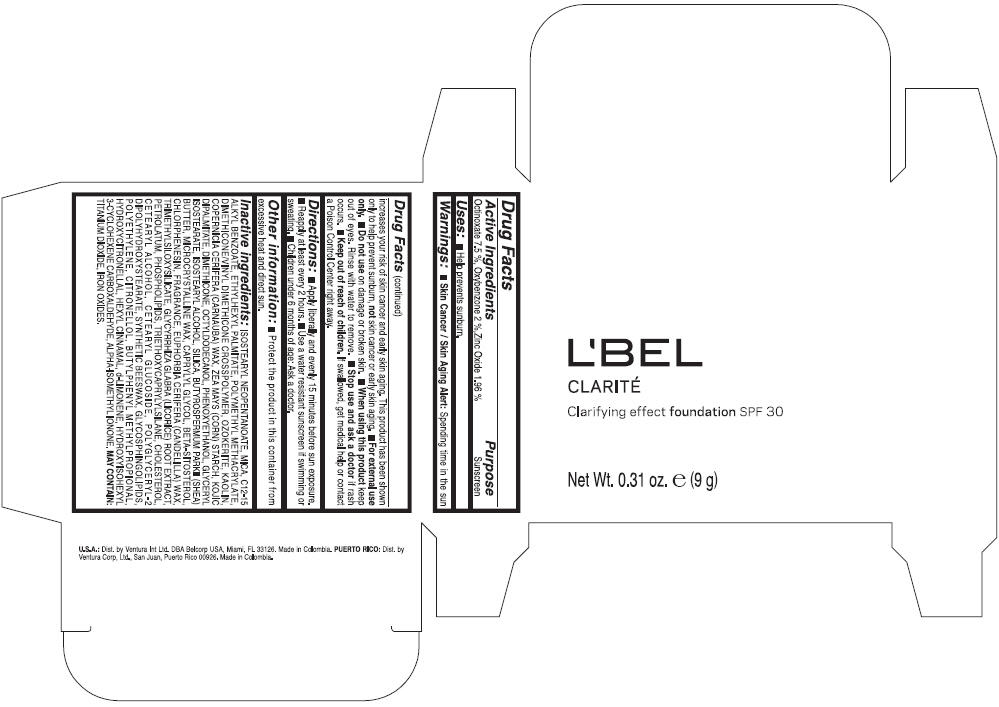
PRINCIPAL DISPLAY PANEL - 9 g Case Box - MEDIUM 7- BEIGE
L'BEL
CLARITÉ
Clarifying effect foundation SPF 30
Net Wt. 0.31 oz. e (9 g)
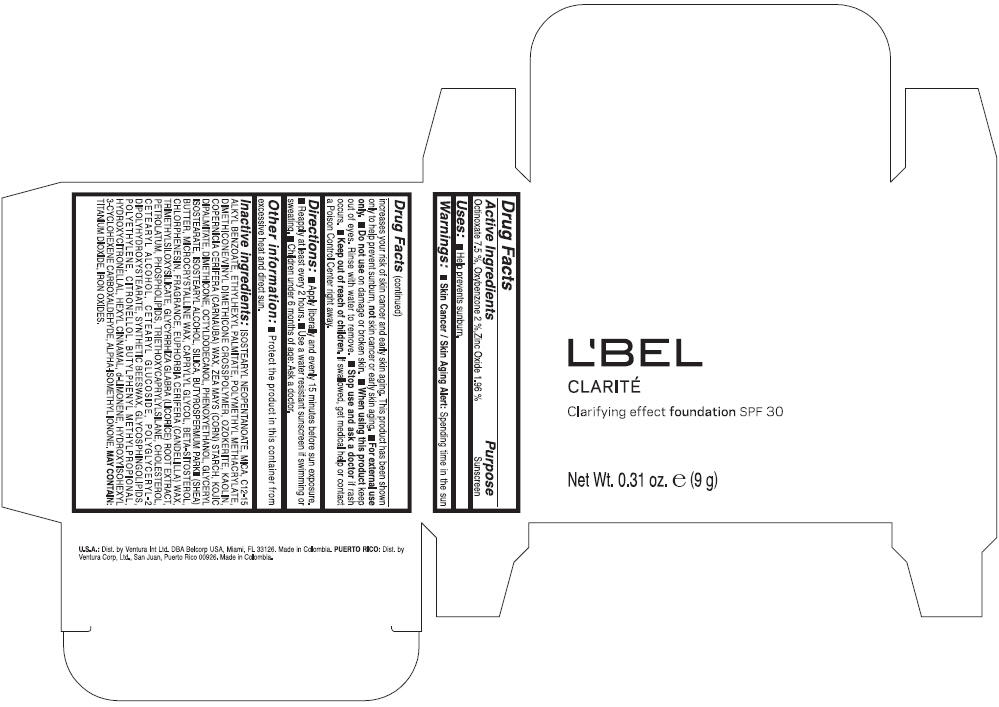
PRINCIPAL DISPLAY PANEL - 9 g Case Box - OBSCURE 8- BROWN
L'BEL
CLARITÉ
Clarifying effect foundation SPF 30
Net Wt. 0.31 oz. e (9 g)