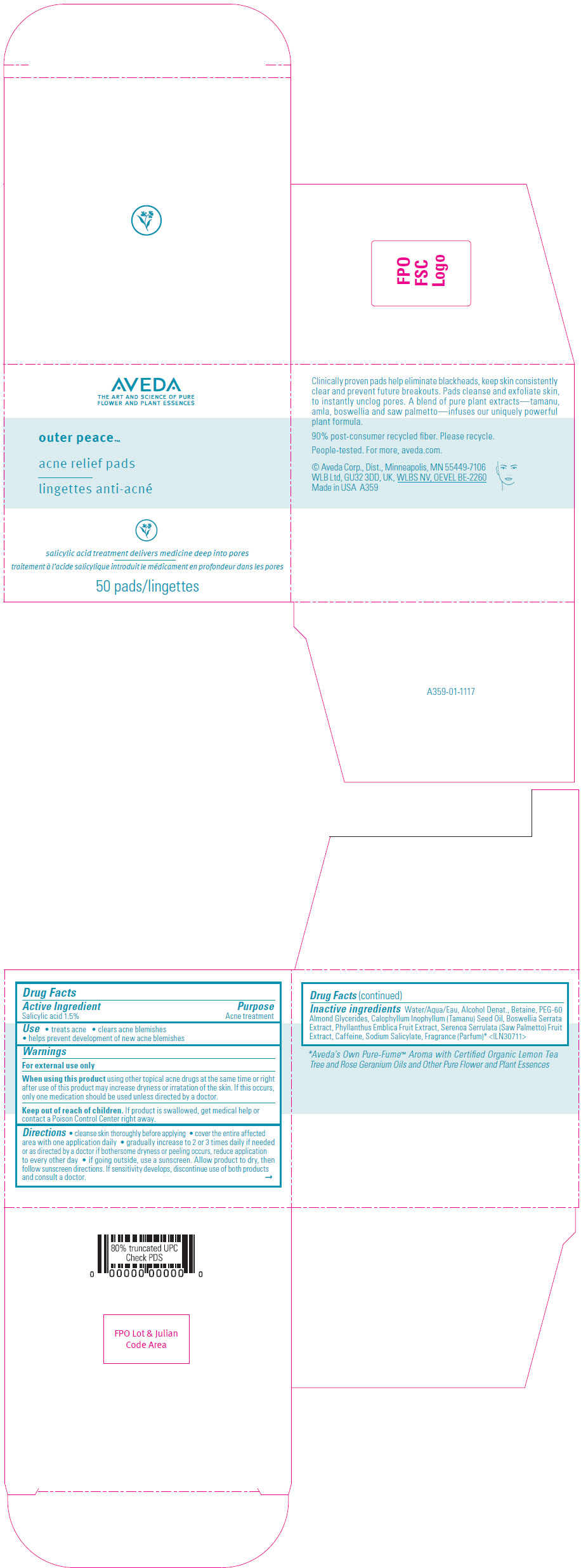
Label: AVEDA OUTER PEACE ACNE RELIEF PADS- salicylic acid solution
- NDC Code(s): 57677-064-01
- Packager: Aveda Corporation
- Category: HUMAN OTC DRUG LABEL
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated July 25, 2023
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active Ingredient
- Purpose
- Use
- Warnings
-
Directions
- cleanse skin thoroughly before applying
- cover the entire affected area with one application daily
- gradually increase to 2 or 3 times daily if needed or as directed by a doctor if bothersome dryness or peeling occurs, reduce application to every other day
- if going outside, use a sunscreen. Allow product to dry, then follow sunscreen directions. If sensitivity develops, discontinue use of both products and consult a doctor.
-
Inactive ingredients
Water/Aqua/Eau, Alcohol Denat., Betaine, PEG-60 Almond Glycerides, Calophyllum Inophyllum (Tamanu) Seed Oil, Boswellia Serrata Extract, Phyllanthus Emblica Fruit Extract, Serenoa Serrulata (Saw Palmetto) Fruit Extract, Caffeine, Sodium Salicylate, Fragrance (Parfum) 1 <ILN30711>
- 1
- Aveda's Own Pure-Fume™ Aroma with Certified Organic Lemon Tea Tree and Rose Geranium Oils and Other Pure Flower and Plant Essences
- PRINCIPAL DISPLAY PANEL - 50 Pad Jar Carton
-
INGREDIENTS AND APPEARANCE
AVEDA OUTER PEACE ACNE RELIEF PADS
salicylic acid solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:57677-064 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SALICYLIC ACID (UNII: O414PZ4LPZ) (SALICYLIC ACID - UNII:O414PZ4LPZ) SALICYLIC ACID 15 mg in 1 g Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) ALCOHOL (UNII: 3K9958V90M) BETAINE (UNII: 3SCV180C9W) PEG-60 ALMOND GLYCERIDES (UNII: 4Y0E651N0F) TAMANU OIL (UNII: JT3LVK84A1) INDIAN FRANKINCENSE (UNII: 4PW41QCO2M) PHYLLANTHUS EMBLICA FRUIT (UNII: YLX4CW2576) SAW PALMETTO (UNII: J7WWH9M8QS) CAFFEINE (UNII: 3G6A5W338E) SODIUM SALICYLATE (UNII: WIQ1H85SYP) FRAGRANCE CLEAN ORC0600327 (UNII: 329LCV5BTF) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:57677-064-01 1 in 1 CARTON 05/17/2021 1 50 g in 1 JAR; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final M006 05/17/2021 Labeler - Aveda Corporation (071352058) Registrant - Estee Lauder Companies Inc. (790802086) Establishment Name Address ID/FEI Business Operations Port Jervis Laboratories, Inc 001535103 manufacture(57677-064)